ADT 2002 Annual Report Download - page 91
Download and view the complete annual report
Please find page 91 of the 2002 ADT annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.TYCO INTERNATIONAL LTD.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (continued)
6. Charges for the Impairment of Long-Lived Assets (continued)
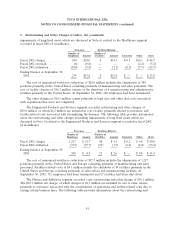
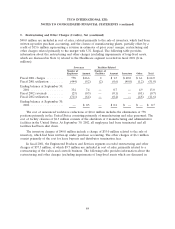
2000 Charges
The Healthcare and Specialty Products segment recorded a charge of $99.0 million in Fiscal 2000
primarily related to an impairment in goodwill and other intangible assets associated with the Company
exiting the interventional cardiology business of U.S. Surgical discussed in Note 5.
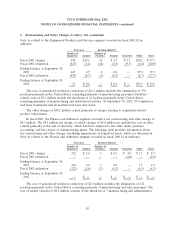
7. Write-Off of Purchased In-Process Research and Development
During fiscal 2002, in connection with Tyco’s acquisition of Sensormatic and DSC Group, the
Company wrote-off the fair value of purchased in-process research and development (‘‘IPR&D’’) of
various projects for the development of new products and technologies in the amount of $17.8 million.
Management determined the value of the IPR&D using, among other factors, appraisals.
In connection with Tyco’s acquisition of Mallinckrodt Inc. during fiscal 2001, the Company
wrote-off the fair value of purchased IPR&D of various projects for the development of new products
and technologies in the amount of $184.3 million. Management determined the valuation of the
IPR&D using, among other factors, appraisals. The value was based primarily on the discounted cash
flow method. This valuation included consideration of (i) the stage of completion of each of the
projects, (ii) the technological feasibility of each of the projects, (iii) whether the projects had an
alternative future use, and (iv) the estimated future residual cash flows that could be generated from
the various projects and technologies over their respective projected economic lives.
As of the Mallinckrodt acquisition date, there were several projects under development at different
stages of completion. The primary basis for determining the technological feasibility of these projects
was obtaining Food and Drug Administration (‘‘FDA’’) approval. As of the acquisition date, none of
the IPR&D projects had received FDA approval. In assessing the technological feasibility of a project,
consideration was also given to the level of complexity and future technological hurdles that each
project had to overcome prior to being submitted to the FDA for approval. As of the acquisition date,
none of the IPR&D projects was considered to be technologically feasible or to have any alternative
future use.
Future residual cash flows that could be generated from each of the projects were determined
based upon management’s estimate of future revenue and expected profitability of the various products
and technologies involved. These projected cash flows were then discounted to their present values
taking into account management’s estimate of future expenses that would be necessary to bring the
projects to completion. The discount rates include a rate of return, which accounts for the time value
of money, as well as risk factors that reflect the economic risk that the cash flows projected may not be
realized. The cash flows were discounted at discount rates ranging from 14% to 25% per annum,
depending on the project’s stage of completion and the type of FDA approval needed. This discounted
cash flow methodology for the various projects included in the purchased IPR&D resulted in a total
valuation of $184.3 million. Although work on the projects related to the IPR&D continued after the
acquisition, the amount of purchase price allocated to IPR&D was written off because the projects
underlying the IPR&D that was being developed were not considered technologically feasible as of the
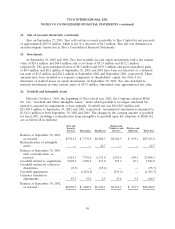
acquisition date. As of September 30, 2002, approximately 44% of the IPR&D projects have been
successfully completed and approximately 30% of the projects have been discontinued or are currently
inactive. The remainder are in various stages of completion. There are currently no expected material
variations between projected results from the projects versus those at the time of the acquisition.
89