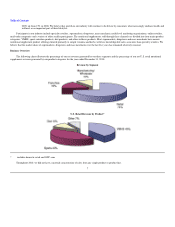
GNC 2011 Annual Report Download - page 19
Download and view the complete annual report
Please find page 19 of the 2011 GNC annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Table of Contents
covered by some of Numico's insurance policies. Following the completion of the Numico acquisition, we obtained our own insurance policies to replace
those Numico policies, including policies for general product liability. We currently maintain product liability insurance and general liability insurance.
We face an inherent risk of exposure to product liability claims in the event that, among other things, the use of products sold by GNC results in injury.
With respect to product liability coverage, we carry insurance coverage typical of our industry and product lines. Our coverage involves self-insured
retentions with primary and excess liability coverage above the retention amount. We have the ability to refer claims to most of our vendors and their insurers
to pay the costs associated with any claims arising from such vendors' products. In most cases, our insurance covers such claims that are not adequately
covered by a vendor's insurance and provides for excess secondary coverage above the limits provided by our product vendors.
We self-insure certain property and casualty risks due to our analysis of the risk, the frequency and severity of a loss and the cost of insurance for the
risk. We believe that the amount of self-insurance is not significant and will not have an adverse impact on our performance. In addition, we may from time to
time self-insure liability with respect to specific ingredients in products that we may sell.
Government Regulation
Product Regulation
Domestic
The processing, formulation, safety, manufacturing, packaging, labeling, advertising and distribution of our products are subject to regulation by one or
more federal agencies, including the Federal Drug Administration (the "FDA"), the FTC, the Consumer Product Safety Commission, the United States
Department of Agriculture (the "USDA") and the Environmental Protection Agency (the "EPA"), and by various agencies of the states and localities in which
our products are sold.
The Dietary Supplement Health and Education Act of 1994 ("DSHEA") established a new framework governing the composition, safety, labeling,
manufacturing and marketing of dietary supplements. Generally, under DSHEA, dietary ingredients that were marketed in the United States prior to
October 15, 1994 may be used in dietary supplements without notifying the FDA. "New" dietary ingredients (i.e., dietary ingredients that were "not marketed
in the United States before October 15, 1994") must be the subject of a new dietary ingredient notification submitted to the FDA unless the ingredient has
been "present in the food supply as an article used for food" without being "chemically altered". A new dietary ingredient notification must provide the FDA
evidence of a "history of use or other evidence of safety" establishing that use of the dietary ingredient "will reasonably be expected to be safe". A new dietary
ingredient notification must be submitted to the FDA at least 75 days before the initial marketing of the new dietary ingredient. The FDA may determine that
a new dietary ingredient notification does not provide an adequate basis to conclude that a dietary ingredient is reasonably expected to be safe. Such a
determination could prevent the marketing of such dietary ingredient. The FDA has announced that it plans to issue a guidance governing notification of new
dietary ingredients. While it is not mandatory to comply with FDA guidance, it is a strong indication of the FDA's current views on the topic of the guidance,
including its position on enforcement. Depending upon the recommendations made in the guidance, particularly those relating to animal or human testing,
such guidance could make it more difficult for us to successfully provide notification of new dietary ingredients.
The Dietary Supplement Safety Act (S 3002), introduced in February 2010, would repeal the provision of DSHEA that permits the sale of all dietary
ingredients sold in dietary supplements
17