Quest Diagnostics 2015 Annual Report Download - page 96
Download and view the complete annual report
Please find page 96 of the 2015 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS – CONTINUED
(in millions unless otherwise indicated)
F- 21
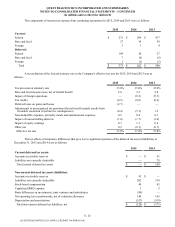
Sale of Royalty Rights
As part of its acquisition of Celera in 2011, the Company gained rights to receive royalties on ibrutinib, an
experimental cancer therapy. In July 2013, the Company sold its right to receive royalties related to the commercialization of
ibrutinib for $485 million in cash. The Company has accounted for this transaction as a sale of royalty rights and recognized a
pre-tax gain of $474 million, net of transaction costs, associated with this sale.
Sale of Enterix
In September 2013, the Company completed the sale of Enterix and recorded a pre-tax loss of approximately $40
million associated with the sale, which is included in other operating expense (income), net. The Enterix business was not
reclassified to discontinued operations due to the level of continuing involvement in the Enterix business subsequent to its sale.
The continuing involvement relates to a minimum purchase agreement between the acquirer of the Enterix business and the
Company.
Sale of HemoCue
In April 2013, the Company completed the sale of HemoCue, which was included in discontinued operations since
2012. For further details regarding the sale of HemoCue, see Note 18.
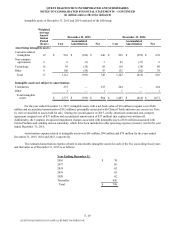
Held for Sale
Non-core Assets Held for Sale
During the third quarter of 2015, the Company classified certain non-core assets as held for sale. The assets consist of
$113 million of goodwill, with the remainder consisting of property, plant and equipment, inventories and intangible assets and
are classified as current assets held for sale in the consolidated balance sheet as of December 31, 2015.
The non-core assets held for sale are included in all other operating segments and have not been classified as
discontinued operations. For further details regarding business segment information, see Note 19.
QUEST DIAGNOSTICS 2015 ANNUAL REPORT ON FORM 10-K