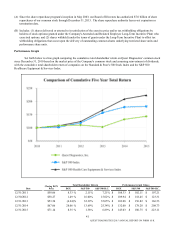
Quest Diagnostics 2015 Annual Report Download - page 39
Download and view the complete annual report
Please find page 39 of the 2015 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.35
Failure to develop, or acquire licenses for, new tests, technology and services could negatively impact our testing volume
and revenues.
The clinical testing industry is faced with changing technology and new product introductions. Other companies or
individuals, including our competitors, may obtain patents or other property rights that would prevent, limit or interfere with
our ability to develop, perform or sell our solutions or operate our business or increase our costs. In addition, they could
introduce new tests, technologies or services that may result in a decrease in the demand for our services or cause us to reduce
the prices of our services. Our success in continuing to introduce new solutions, technology and services will depend, in part,
on our ability to license new and improved technologies on favorable terms. We may be unable to develop or introduce new
solutions or services. We also may be unable to continue to negotiate acceptable licensing arrangements, and arrangements that
we do conclude may not yield commercially successful clinical tests. If we are unable to license these testing methods at
competitive rates, our research and development costs may increase as a result. In addition, if we are unable to develop and
introduce, or license, new solutions, technology and services to expand our esoteric testing business, our services may become
outdated when compared with our competition.
We may be unable to obtain, maintain or enforce our intellectual property rights and may be subject to intellectual
property litigation that could adversely impact our business.
We may be unable to obtain or maintain adequate patent or other proprietary rights for our solutions or services or to
successfully enforce our proprietary rights. In addition, we may be subject to intellectual property litigation and we may be
found to infringe on the proprietary rights of others, which could force us to do one or more of the following:
• cease developing, performing or selling solutions or services that incorporate the challenged intellectual property;
• obtain and pay for licenses from the holder of the infringed intellectual property right;
• redesign or re-engineer our tests;
• change our business processes; or
• pay substantial damages, court costs and attorneys' fees, including potentially increased damages for any
infringement held to be willful.
The development of new, more cost-effective solutions that can be performed by our customers or by patients, and the
continued internalization of testing by hospitals or physicians, could negatively impact our testing volume and revenues.
The diagnostic information services industry is faced with changing technology and new product introductions,
including technology that enables more convenient or cost-effective testing. Competitors also may offer testing to be
performed outside of a commercial clinical laboratory, such as (1) point-of-care testing that can be performed by physicians in
their offices; (2) complex testing that can be performed by hospitals in their own laboratories; and (3) home testing that can be
carried out without requiring the services of outside providers. Advances in technology also may lead to the need for less
frequent testing. Further, diagnostic tests approved or cleared by the FDA for home use are automatically deemed to be
“waived” tests under CLIA and may be performed by patients in their homes; test kit manufacturers could seek to increase sales
to patients of such test kits.
Some traditional customers for anatomic pathology services, including specialty physicians that generate biopsies
through surgical procedures, such as dermatologists, gastroenterologists, urologists and oncologists, have added in-office
histology labs or have retained pathologists to read cases on site. Hospitals also are internalizing clinical laboratory testing,
including some esoteric testing. Internalization of testing may reduce demand for services previously referred to outside
service providers, such as the Company.
Our outstanding debt may impair our financial and operating flexibility.
As of December 31, 2015, we had approximately $3.7 billion of debt outstanding. Except for operating leases, we do
not have any off-balance sheet financing arrangements in place or available. Our debt agreements contain various restrictive
covenants. These restrictions could limit our ability to use operating cash flow in other areas of our business because we must
use a portion of these funds to make principal and interest payments on our debt. We have obtained ratings on our debt from
Standard and Poor's, Moody's Investor Services and Fitch Ratings. There can be no assurance that any rating so assigned will
remain for any given period of time or that a rating will not be lowered or withdrawn entirely by a rating agency if in that rating
agency's judgment future circumstances relating to the basis of the rating, such as adverse changes in our Company or our
industry, so warrant. If such ratings are lowered, our borrowing costs could increase. Changes in our credit ratings, however, do
not require repayment or acceleration of any of our debt.
QUEST DIAGNOSTICS 2015 ANNUAL REPORT ON FORM 10-K