Quest Diagnostics 2015 Annual Report Download - page 59
Download and view the complete annual report
Please find page 59 of the 2015 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.55
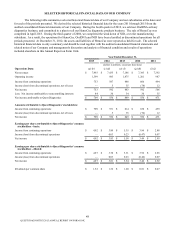
In November 2015, we announced that we entered into a definitive agreement to acquire the outreach laboratory
service business of Clinical Laboratory Partners ("CLP"), a wholly-owned subsidiary of Hartford HealthCare. CLP provides
clinical lab testing to physicians in Connecticut. The acquisition is expected to be completed in the first quarter of 2016,
subject to customary regulatory closing conditions.
Contribution of Clinical Trials Business
On July 1, 2015, we closed on our joint venture with Quintiles to form a global clinical trials central laboratory
services joint venture, Q2 Solutions. In connection with the transaction, we contributed certain assets of our clinical trials
testing business to the newly formed joint venture in exchange for a non-controlling, 40% ownership interest ("Clinical Trials
Contribution"). As a result of the transaction, we recognized a non-cash pre-tax gain of $334 million. The Clinical Trials
Contribution is consistent with our five-point strategy.
Consolidated net revenues and operating costs and expenses include the operating results of our clinical trials testing
business prior to closing. Subsequent to closing, our ownership interest in the operating results of the joint venture is being
accounted for under the equity method of accounting and recorded within a single line item, equity in earnings of equity
method investees, net of taxes, on the consolidated statements of operations.
Assets Held for Sale
During the third quarter of 2015, certain non-core assets of our DS businesses were reclassified to assets held for sale.
For further details regarding our dispositions and assets held for sale, see Note 6 to the consolidated financial
statements.
Senior Notes Offering
In March 2015, we completed a $1.2 billion senior notes offering (the "2015 Senior Notes") that was sold in three
tranches: (a) $300 million aggregate principal amount of 2.50% senior notes due March 2020, issued at a discount of $1
million; (b) $600 million aggregate principal amount of 3.50% senior notes due March 2025; and (c) $300 million aggregate
principal amount of 4.70% senior notes due March 2045. A portion of the proceeds from the 2015 Senior Notes were used to
fund the cash tender offer (the "Tender Offer") in March 2015 in which we purchased $176 million of our 6.95% Senior Notes
due July 2037 and $74 million of our 5.75% Senior Notes due January 2040. The remaining proceeds from the 2015 Senior
Notes, together with borrowing under our existing credit facilities, were used in April 2015 to redeem all of our $500 million
5.45% Senior Notes due November 2015, $150 million, or 50%, of our 3.2% Senior Notes due April 2016 and all of our $375
million 6.4% Senior Notes due July 2017 (the "Redemption"). In connection with the Tender Offer and Redemption, we
recorded a $79 million pre-tax loss on early retirement of debt in the first quarter of 2015 and a $65 million pre-tax loss on
early retirement of debt in the second quarter of 2015, respectively.
For further details regarding our 2015 Senior Notes, Tender Offer and Redemption, see Note 13 to the consolidated
financial statements.
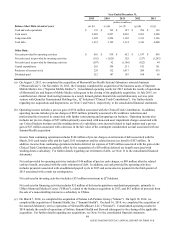
Critical Accounting Policies
The preparation of financial statements in conformity with accounting principles generally accepted in the United
States requires us to make estimates and assumptions and select accounting policies that affect our reported financial results
and the disclosure of contingent assets and liabilities.
While many operational aspects of our business are subject to complex federal, state and local regulations, the
accounting for most of our business is generally straightforward, with net revenues primarily recognized upon completion of
the testing process. Our revenues are primarily comprised of a high volume of relatively low-dollar transactions, and about
one-half of our total costs and expenses consist of employee compensation and benefits. Due to the nature of our business,
several of our accounting policies involve significant estimates and judgments:
• revenues and accounts receivable associated with DIS;
• reserves for general and professional liability claims;
• reserves for other legal proceedings;
• accounting for and recoverability of goodwill; and
• accounting for stock-based compensation expense.
QUEST DIAGNOSTICS 2015 ANNUAL REPORT ON FORM 10-K