Quest Diagnostics 2015 Annual Report Download - page 74
Download and view the complete annual report
Please find page 74 of the 2015 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.70
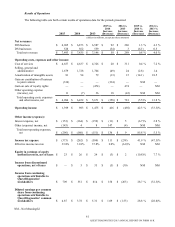
Equity Method Investees
Our equity method investees primarily consist of our clinical trials central laboratory services joint venture and our
diagnostic information services joint ventures, which are accounted for under the equity method of accounting. We believe that
our transactions with our equity method investees are conducted at arm’s length, reflecting current market conditions and
pricing. Our investment in equity method investees equals less than 5% of our consolidated total assets. Our proportionate
share of income before income taxes associated with our equity method investees equals less than 5% of our consolidated
income before income taxes and equity in earnings of equity method investees. We have no material unconditional obligations
or guarantees to, or in support of, our equity method investees and their operations.
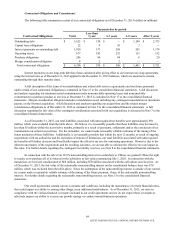
Requirements and Capital Resources
We estimate that we will invest approximately $250 million to $300 million during 2016 for capital expenditures, to
support and grow our existing operations, principally related to investments in information technology, laboratory equipment
and facilities, including specific initiatives associated with our Invigorate and other programs.
We believe our cash on hand, cash from operations and borrowing capacity under our credit facilities are sufficient to
fund the repayment of the current portion of our long-term debt and the CLP acquisition (discussed in "Recent Transactions:
Acquisitions").
In October 2015, we amended and restated the $525 million secured receivables credit facility, increasing the
borrowing capacity to $600 million. The amended and restated secured receivables credit facility matures in October 2017.
Under the credit facility, we can issue letters of credit totaling $100 million. Issued letters of credit reduce the available
borrowing capacity under the facility. For further details regarding the outstanding letters of credit, see Note 17 to the
consolidated financial statements.
As of December 31, 2015, $1.3 billion of borrowing capacity was available under our secured receivables credit
facility and senior unsecured revolving credit facility. Should one or several banks no longer participate in either of our credit
facilities, we would not expect it to impact our ability to fund operations. We expect that we will be able to replace our existing
credit facilities with alternative arrangements prior to their expiration. For further details regarding the credit facilities, see
Note 13 to the consolidated financial statements.
At December 31, 2015, approximately 37% of our $133 million of consolidated cash and cash equivalents were held
outside of the United States. These funds are considered indefinitely reinvested to be used to expand operations either
organically or through acquisitions outside the United States. Further, our current plans do not demonstrate a need to repatriate
foreign funds in order to fund operations in the United States. If the foreign cash and cash items are needed for operations in
the United States, or we otherwise elect to repatriate the funds, we may be required to accrue and pay United States taxes on a
significant portion of these amounts.
We believe that cash and cash equivalents on-hand and cash from operations, together with our borrowing capacity
under our credit facilities, will provide sufficient financial flexibility to fund seasonal and other working capital requirements,
capital expenditures, debt service requirements and other obligations, cash dividends on common shares, share repurchases and
additional growth opportunities for the foreseeable future. We believe that our credit profile should provide us with access to
additional financing to refinance upcoming debt maturities and, if necessary, to fund growth opportunities that cannot be
funded from existing sources.
Inflation
We believe that inflation generally does not have a material adverse effect on our results of operations or financial
condition.
Impact of New Accounting Standards
The impacts of recent accounting pronouncements not yet effective on our consolidated financial statements are
discussed in Note 2 to the consolidated financial statements.
QUEST DIAGNOSTICS 2015 ANNUAL REPORT ON FORM 10-K