Quest Diagnostics 2015 Annual Report Download - page 34
Download and view the complete annual report
Please find page 34 of the 2015 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
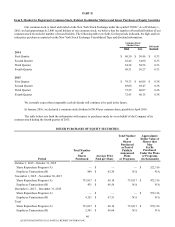
30
Michael E. Prevoznik (54)
Senior Vice President and General
Counsel
Mr. Prevoznik joined the Company as Vice President and General
Counsel in August 1999. In 2003, he assumed responsibility for
governmental affairs. From 1999 until April 2009, Mr. Prevoznik also
had responsibility for the Company's Compliance Department.
Since April 2011, in addition to serving as General Counsel, Mr.
Prevoznik has had management responsibility for the Company's
diagnostic information services activities outside the U.S. In addition,
from April 2011 to January 2013, Mr. Prevoznik had management
responsibility for the Company's clinical trials business.
Prior to joining the Company, Mr. Prevoznik served in positions of
increasing responsibility within the compliance organization at
SmithKline Beecham, most recently as Vice President, Compliance,
with responsibility for coordinating all SmithKline Beecham
compliance activities worldwide.
Item 1A. Risk Factors
You should carefully consider all of the information set forth in this Report, including the following risk factors,
before deciding to invest in any of our securities. The risks below are not the only ones that we face. Additional risks not
presently known to us, or that we presently deem immaterial, may also negatively impact us. Our business, consolidated
financial condition, revenues, results of operations, profitability, reputation or cash flows could be materially impacted by any
of these factors.
The U.S. healthcare system is evolving, and our business could be adversely impacted if we fail to adapt.
The U.S. healthcare system is evolving, in part in response to the passage of the Affordable Care Act ("ACA") in
2010. The law provided for reductions in the Medicare clinical laboratory fee schedule of 1.75% for five years beginning in
2011 and also included a productivity adjustment that reduced the CPI market basket update since 2011. The law imposes an
excise tax on the seller for the sale of certain medical devices in the U.S., including those purchased and used by laboratories;
effective January 2016, Congress imposed a two-year moratorium on the device tax. The law established the Independent
Payment Advisory Board, which is responsible to submit annually proposals aimed at reducing Medicare cost growth while
preserving quality. These proposals automatically will be implemented unless Congress enacts alternative proposals that
achieve the same savings targets. Further, the ACA established the Center for Medicare and Medicaid Innovation to examine
alternative payment methodologies and conduct demonstration programs. The law provided for extensive health insurance
reforms, including the elimination of pre-existing condition exclusions and other limitations on coverage, fixed percentages on
medical loss ratios, expansion in Medicaid and other programs, employer mandates, individual mandates, creation of state and
regional health insurance exchanges, and tax subsidies for individuals to help cover the cost of individual insurance coverage.
The law also permits the establishment of ACOs.
Significant change is taking place in the healthcare system, including as discussed above under the heading The
United States Clinical Testing Industry, beginning on page 14. For example, ACOs and patient-centered medical homes are
growing as a means to deliver patient care. Value-based reimbursement is increasing; CMS has set goals for value-based
reimbursement to be achieved in coming years. Healthcare industry participants are consolidating. Healthcare services
increasingly are being provided by non-traditional providers (e.g., physician assistants), in non-traditional venues (e.g., retail
medical clinics, urgent care centers) and using new technologies (e.g., telemedicine). We expect that the evolution of the
healthcare industry will continue, and that industry change is likely to be extensive.
This Report also includes forward-looking statements that involve risks or uncertainties. Our results could differ
materially from those anticipated in these forward-looking statements as a result of certain factors, including the risks we face
described below and elsewhere. See “Cautionary Factors that May Affect Future Results” on page 37.
QUEST DIAGNOSTICS 2015 ANNUAL REPORT ON FORM 10-K