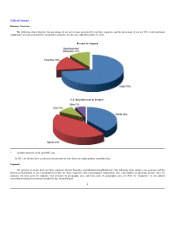
GNC 2012 Annual Report Download - page 20
Download and view the complete annual report
Please find page 20 of the 2012 GNC annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Table of Contents
The FDC Act permits "statements of nutritional support" to be included in labeling for dietary supplements without FDA pre-market approval. Such
statements must be submitted to the FDA within 30 days of marketing. Such statements may describe how a particular dietary ingredient affects the structure,
function or general well-being of the body, or the mechanism of action by which a dietary ingredient may affect body structure, function or well-being, but
may not expressly or implicitly represent that a dietary supplement will diagnose, cure, mitigate, treat or prevent a disease. A company that uses a statement of
nutritional support in labeling must possess scientific evidence substantiating that the statement is truthful and not misleading. If the FDA determines that a
particular statement of nutritional support is an unacceptable drug claim, conventional food claim or an unauthorized version of a "health claim," or, if the
FDA determines that a particular claim is not adequately supported by existing scientific data or is false or misleading, we would be prevented from using the
claim.
In addition, DSHEA provides that so-called "third-party literature," e.g., a reprint of a peer-reviewed scientific publication linking a particular dietary
ingredient with health benefits, may be used "in connection with the sale of a dietary supplement to consumers" without the literature being subject to
regulation as labeling. The literature: (1) must not be false or misleading; (2) may not "promote" a particular manufacturer or brand of dietary supplement;
(3) must present a balanced view of the available scientific information on the subject matter; (4) if displayed in an establishment, must be physically separate
from the dietary supplements; and (5) should not have appended to it any information by sticker or any other method. If the literature fails to satisfy each of
these requirements, we may be prevented from disseminating such literature with our products, and any dissemination could subject our product to regulatory
action as an illegal drug.
In June 2007, pursuant to the authority granted by the FDC Act as amended by DSHEA, the FDA published detailed current Good Manufacturing
Practice ("cGMP") regulations that govern the manufacturing, packaging, labeling and holding operations of dietary supplement manufacturers. The cGMP
regulations, among other things, impose significant recordkeeping requirements on manufacturers. The cGMP requirements are in effect for all manufacturers,
and the FDA is conducting inspections of dietary supplement manufacturers pursuant to these requirements. There remains considerable uncertainty with
respect to the FDA's interpretation of the regulations and their actual implementation in manufacturing facilities. In addition, the FDA's interpretation of the
regulations will likely change over time as the agency becomes more familiar with the industry and the regulations. The failure of a manufacturing facility to
comply with the cGMP regulations renders products manufactured in such facility "adulterated," and subjects such products and the manufacturer to a variety
of potential FDA enforcement actions. In addition, under the Food Safety Modernization Act ("FSMA"), which was enacted on January 2, 2011, the
manufacturing of dietary ingredients contained in dietary supplements will be subject to similar or even more burdensome manufacturing requirements, which
will likely increase the costs of dietary ingredients and will subject suppliers of such ingredients to more rigorous inspections and enforcement. The FSMA
will also require importers of food, including dietary supplements and dietary ingredients, to conduct verification activities to ensure that the food they might
import meets applicable domestic requirements.
The FDA has broad authority to enforce the provisions of federal law applicable to dietary supplements, including powers to issue a public warning or
notice of violation letter to a company, publicize information about illegal products, detain products intended for import, require the reporting of serious
adverse events, require a recall of illegal or unsafe products from the market, and request the Department of Justice to initiate a seizure action, an injunction
action or a criminal prosecution in the U.S. courts. The FSMA expands the reach and regulatory powers of the FDA with respect to the production and
importation of food, including dietary supplements. The expanded reach and regulatory powers include the FDA's ability to order mandatory recalls,
administratively detain domestic products, require certification of compliance with domestic requirements for imported foods associated with safety issues
and administratively revoke manufacturing facility registrations, effectively enjoining manufacturing of
18