GNC 2012 Annual Report Download - page 100
Download and view the complete annual report
Please find page 100 of the 2012 GNC annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Table of Contents
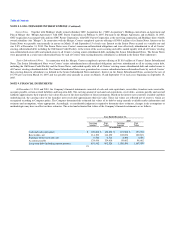
NOTE 11. COMMITMENTS AND CONTINGENCIES
Litigation
The Company is engaged in various legal actions, claims and proceedings arising in the normal course of business, including claims related to breach of
contracts, products liabilities, intellectual property matters and employment-related matters resulting from the Company's business activities. As with most
actions such as these, an estimation of any possible and/or ultimate liability cannot always be determined. The Company continues to assess the requirement
to account for additional contingencies in accordance with the standard on contingencies. If the Company is required to make a payment in connection with an
adverse outcome in these matters, it could have a material adverse effect on the Company's business, financial condition, results of operations or cash flows.
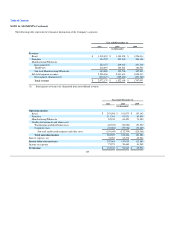
As a manufacturer and retailer of nutritional supplements and other consumer products that are ingested by consumers or applied to their bodies, the
Company has been and is currently subjected to various product liability claims. Although the effects of these claims to date have not been material to the
Company, it is possible that current and future product liability claims could have a material adverse effect on its business or financial condition, results of
operations or cash flows. The Company currently maintains product liability insurance with a deductible/retention of $3.0 million per claim with an aggregate
cap on retained loss of $10.0 million. The Company typically seeks and has obtained contractual indemnification from most parties that supply raw materials
for its products or that manufacture or market products it sells. The Company also typically seeks to be added, and has been added, as an additional insured
under most of such parties' insurance policies. The Company is also entitled to indemnification by Numico for certain losses arising from claims related to
products containing ephedra or Kava Kava sold prior to December 5, 2003. However, any such indemnification or insurance is limited by its terms and any
such indemnification, as a practical matter, is limited to the creditworthiness of the indemnifying party and its insurer, and the absence of significant defenses
by the insurers. The Company may incur material products liability claims, which could increase its costs and adversely affect its reputation, revenues and
operating income.
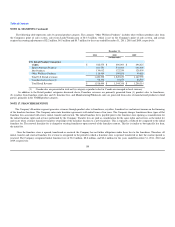
Hydroxycut Claims. On May 1, 2009, the FDA issued a warning on several Hydroxycut-branded products manufactured by Iovate Health Sciences
U.S.A., Inc. ("Iovate"). The FDA warning was based on 23 reports of liver injuries from consumers who claimed to have used the products between 2002 and
2009. As a result, Iovate voluntarily recalled 14 Hydroxycut-branded products.
Following the recall, the Company was named, among other defendants, in approximately 85 lawsuits related to Hydroxycut-branded products in 13
states. Iovate previously accepted the Company's tender request for defense and indemnification under its purchasing agreement with the Company and, as
such, Iovate has accepted the Company's request for defense and indemnification in the Hydroxycut matters. The Company's ability to obtain full recovery in
respect of any claims against the Company in connection with products manufactured by Iovate under the indemnity is dependent on Iovate's insurance
coverage, the creditworthiness of its insurer, and the absence of significant defenses by such insurer. To the extent the Company is not fully compensated by
Iovate's insurer, it can seek recovery directly from Iovate. The Company's ability to fully recover such amounts may be limited by the creditworthiness of
Iovate.
As of December 31, 2011, there were 75 pending lawsuits related to Hydroxycut in which the Company had been named: 69 individual, largely personal
injury claims and six putative class action cases, generally inclusive of claims of consumer fraud, misrepresentation, strict liability and breach of warranty.
Any liabilities that may arise from these matters are not probable or reasonably estimable at this time.
By court order dated October 6, 2009, the United States Judicial Panel on Multidistrict Litigation consolidated pretrial proceedings of many of the
pending actions in the Southern District of California (In re: Hydroxycut Marketing and Sales Practices Litigation, MDL No. 2087).
95