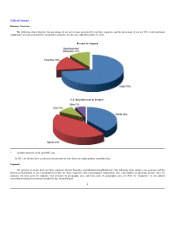
GNC 2012 Annual Report Download - page 19
Download and view the complete annual report
Please find page 19 of the 2012 GNC annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Table of Contents
and potential penalties associated with manufacturing and selling dietary supplements, and reduce our growth prospects.
The Dietary Supplement Safety Act (S 3002), introduced in February 2010, would repeal the provision of DSHEA that permits the sale of all dietary
ingredients sold in dietary supplements marketed in the United States prior to October 15, 1994, and instead permit the sale of only those dietary ingredients
included on a list of Accepted Dietary Ingredients to be issued and maintained by the FDA. The bill also would allow the FDA to: impose a fine of twice the
gross profits earned by a distributor on sales of any dietary supplement found to violate the law; require a distributor to submit a yearly report on all non-
serious Adverse Event Reports ("AERs") received during the year to the FDA; and allow the FDA to recall any dietary supplement it determines with "a
reasonable probability" would cause serious adverse health consequences or is adulterated or misbranded. The bill also would require any dietary supplement
distributor to register with the FDA and submit a list of the ingredients in and copies of the labels of its dietary supplements to the FDA and thereafter update
such disclosures yearly and submit any new dietary supplement product labels to the FDA before marketing any dietary supplement product. If this bill is
reintroduced and enacted, it could severely restrict the number of dietary supplements available for sale and increase our costs and potential penalties
associated with selling dietary supplements.
The FDA or other agencies could take actions against products or product ingredients that in its determination present an unreasonable health risk to
consumers that would make it illegal for us to sell such products. In addition, the FDA could issue consumer warnings with respect to the products or
ingredients in such products that are sold in our stores. Such actions or warnings could be based on information received through FDC Act-mandated
reporting of serious adverse events. For example, the FDC Act requires that reports of serious adverse events be submitted to the FDA, and based in part on
such reports, in May 2009, the FDA warned consumers to stop using Hydroxycut diet products, which are produced by Iovate Health Sciences, Inc. ("Iovate")
and were sold in our stores. Iovate issued a voluntary recall, with which we fully complied. Sales of the recalled Hydroxycut products amounted to
approximately $57.8 million, or 4.7% of our retail sales in 2008, and $18.8 million, or 4.2% of our retail sales in the first four months of 2009. Through
December 31, 2011, we estimate that we had refunded approximately $3.5 million to our retail customers and approximately $1.6 million to our wholesale
customers for Hydroxycut product returns.
As is common in our industry, we rely on our third-party vendors to ensure that the products they manufacture and sell to us comply with all applicable
regulatory and legislative requirements. In general, we seek representations and warranties, indemnification and/or insurance from our vendors. However,
even with adequate insurance and indemnification, any claims of non-compliance could significantly damage our reputation and consumer confidence in our
products. In addition, the failure of such products to comply with applicable regulatory and legislative requirements could prevent us from marketing the
products or require us to recall or remove such products from the market, which in certain cases could materially and adversely affect our business, financial
condition and results of operations. For example, we sell products manufactured by third parties that contain DMAA (as defined below). Although we have
received representations from our third-party vendors that these products comply with applicable regulatory and legislative requirements, recent media articles
have suggested that DMAA may not comply with the FDC Act. In December 2011, the U.S. military asked us to temporarily remove products containing
DMAA from our stores on its bases pending the outcome of a precautionary review. That review is still pending. If it is determined that DMAA does not
comply with applicable regulatory and legislative requirements, we could be required to recall or remove from the market all products containing DMAA and
we could become subject to lawsuits related to any alleged non-compliance, any of which could materially and adversely affect our business, financial
condition and results of operations. In the past, we have attempted to offset any losses related to recalls and removals with reformulated or alternative
products; however, there can be no assurance that we would be able to offset all or any portion of losses related to any future removal or recall.
17