CVS 2015 Annual Report Download - page 95
Download and view the complete annual report
Please find page 95 of the 2015 CVS annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
93
2015 Annual Report
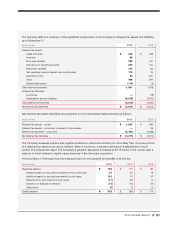
• The Attorney General of the State of Texas issued civil investigative demands and other requests in February
2012, May 2014, and May 2015, and has continued its investigation concerning the Health Savings Pass program
and other pricing practices with respect to claims for reimbursement from the Texas Medicaid program.
• In July and September 2015, two related putative class actions, Corcoran
et al.
v. CVS Health Corp., and
Podgorny
et al.
v. CVS Health Corp., were filed against the Company in the United States District Court in the
Northern District of California and the Northern District of Illinois, respectively. The two cases have been consoli-
dated in United States District Court in the Northern District of California. The consolidated second amended
complaint alleges that plaintiffs overpaid for prescriptions for generic drugs filled at CVS pharmacies. The plaintiffs
seek damages and injunctive relief under the consumer protection statutes and common laws of certain states.
The Company has moved to dismiss the second amended complaint.
• In September 2015, Omnicare was served with an administrative subpoena by the DEA. The subpoena seeks
documents related to controlled substance policies, procedures, and practices at eight pharmacy locations from
May 2012 to present. The Company has been cooperating and providing documents in response to this adminis-
trative subpoena.
• In October 2015, Omnicare received a Civil Investigative Demand from the United States Attorney’s Office for the
Southern District of New York requesting information and documents concerning Omnicare’s cycle fill process for
assisted living facilities. The Company has been cooperating with the government and providing documents and
information in response to the Civil Investigative Demand.
• In October 2015, the Company received from the U.S. Department of Justice a Civil Investigative Demand
requesting documents and information in connection with a False Claims Act investigation concerning allegations
that the Company submitted, or caused to be submitted, to the Medicare Part D program prescription drug event
data that misrepresented true prices paid by the Company’s PBM to pharmacies for drugs dispensed to Part D
beneficiaries with prescription benefits administered by the Company’s PBM. The Company has been cooperating
with the government and providing documents and information in response to the Civil Investigative Demand.
• In November 2015, the United States District Court for the Eastern District of Pennsylvania unsealed a second
amended
qui tam
complaint filed in September 2015, in an action captioned The United States of America
et al.
ex rel.
Sally Schimelpfeinig and John Segura v. Dr. Reddy’s Laboratories Limited
et al.
The U.S. Department of
Justice declined to intervene in this action. The relators allege that the Company, Walgreens, Wal-Mart, and Dr.
Reddy’s Laboratories violated the federal and various state False Claims Acts by dispensing prescriptions in unit
dose packaging supplied by Dr. Reddy’s that was not compliant with the Consumer Product Safety Improvement
Act and the Poison Preventive Packaging Act and thereby allegedly rendering the drugs misbranded under the
Food, Drug & Cosmetic Act.
The Company is also a party to other legal proceedings, government investigations, inquiries and audits arising in
the normal course of its business, none of which is expected to be material to the Company. The Company can give
no assurance, however, that its business, financial condition and results of operations will not be materially adversely
affected, or that the Company will not be required to materially change its business practices, based on: (i) future
enactment of new health care or other laws or regulations; (ii) the interpretation or application of existing laws or
regulations as they may relate to the Company’s business, the pharmacy services, retail pharmacy, long-term care
pharmacy or retail clinic industries or to the health care industry generally; (iii) pending or future federal or state
governmental investigations of the Company’s business or the pharmacy services, retail pharmacy, long-term care
pharmacy or retail clinic industry or of the health care industry generally; (iv) pending or future government enforce-
ment actions against the Company; (v) adverse developments in any pending
qui tam
lawsuit against the Company,
whether sealed or unsealed, or in any future
qui tam
lawsuit that may be filed against the Company; or (vi) adverse
developments in pending or future legal proceedings against the Company or affecting the pharmacy services, retail
pharmacy, long-term care pharmacy or retail clinic industry or the health care industry generally.