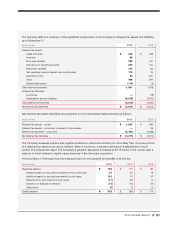
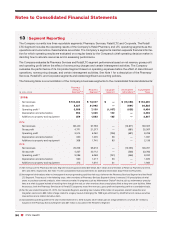
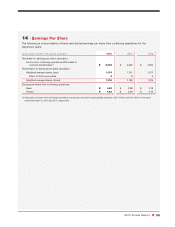
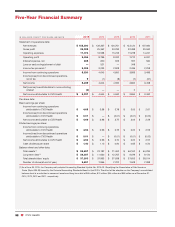
CVS 2015 Annual Report Download - page 93
Download and view the complete annual report
Please find page 93 of the 2015 CVS annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
91
2015 Annual Report
those being investigated at that time by the U.S. Federal Trade Commission (“FTC”). Twenty-eight states, the
District of Columbia and the County of Los Angeles are known to be participating in this investigation. The prior
FTC investigation, which commenced in August 2009, was officially concluded in May 2012 when the consent
order entered into between the FTC and the Company became final. The Company has cooperated with the
multi-state investigation.
• In March 2010, the Company received a subpoena from the OIG requesting information about programs under
which the Company has offered customers remuneration conditioned upon the transfer of prescriptions for drugs
or medications to the Company’s pharmacies in the form of gift cards, cash, non-prescription merchandise or
discounts or coupons for non-prescription merchandise. The subpoena relates to an investigation of possible
false or otherwise improper claims for payment under the Medicare and Medicaid programs. The Company has
provided documents and other information in response to this request for information.
• On October 29, 2010, a
qui tam
complaint entitled United States
et al.
,
ex rel.
Banigan and Templin v. Organon
USA, Inc., Omnicare, Inc. and PharMerica Corporation, Civil No. 07-12153-RWZ, that had been filed under seal
with the U.S. District Court in Boston, Massachusetts, was ordered unsealed by the court. The complaint was
brought by James Banigan and Richard Templin, former employees of Organon, as private party
qui tam
relators
on behalf of the federal government and several state and local governments. The action alleges civil violations of
the False Claims Act based on allegations that Organon and its affiliates paid Omnicare and several other long-
term care pharmacies rebates, post-purchase discounts and other forms of remuneration in return for purchasing
pharmaceuticals from Organon and taking steps to increase the purchase of Organon’s drugs in violation of the
Anti-Kickback Statute. The U.S. Department of Justice declined to intervene in this action. The court denied
Omnicare’s motion to dismiss in June 2012. Discovery is closed in this matter. The Company believes that the
allegations are without merit.
• In January 2012, the United States District Court for the Eastern District of Pennsylvania unsealed a first amended
qui tam
complaint filed in August 2011 by an individual relator, Anthony Spay, who is described in the complaint
as having once been employed by a firm providing pharmacy prescription benefit audit and recovery services.
The complaint seeks monetary damages and alleges that Caremark’s processing of Medicare claims on behalf of
one of its clients violated the federal False Claims Act. The United States declined to intervene in the lawsuit. In
September 2015, the Court granted Caremark’s motion for summary judgment in its entirety, and entered judg-
ment in favor of Caremark and against Spay. In October 2015, Spay filed a notice of appeal in the United States
Court of Appeals for the Third Circuit.
• In November 2012, the Company received a subpoena from the OIG requesting information concerning automatic
refill programs used by pharmacies to refill prescriptions for customers. The Company has been cooperating and
providing documents and other information in response to this request for information.
• In 2013, Omnicare received subpoenas seeking information regarding Omnicare’s nationwide billing practices with
regard to National Drug Code overrides and Omnicare’s May 2008 acquisition of Pure Service Pharmacy. In 2014,
Omnicare received subpoenas seeking information regarding Omnicare’s Auto Label Verification system and
Omnicare’s per diem arrangements. Omnicare has produced documents and provided information in response to
these subpoenas and continues to cooperate in the investigations.
• On March 22, 2013, a
qui tam
complaint entitled United States
et al. ex rel.
Susan Ruscher v. Omnicare, Inc.
et al.
,
Civil No. 08-cv-3396, which had been filed under seal in the U.S. District Court for the Southern District of Texas,
was unsealed by the court. The complaint was brought by Susan Ruscher as a private party
qui tam
relator on
behalf of the federal government and several state governments alleging violations of the federal False Claims
Act and analogous state laws based upon allegations that Omnicare’s practices relating to customer collections
violated the Anti-Kickback Statute. In September 2015, the court granted summary judgment dismissing all claims
against Omnicare and denied relator’s motion for summary judgment related to Omnicare’s counterclaims and
thereafter, in October 2015, the court entered a final judgment for Omnicare and stayed trial on the counterclaims