CVS 2015 Annual Report Download - page 94
Download and view the complete annual report
Please find page 94 of the 2015 CVS annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
92 CVS Health
Notes to Consolidated Financial Statements
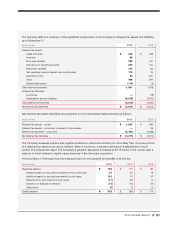
pending an appeal from the relator. In October 2015, plaintiffs filed a notice of appeal in the United States Court of
Appeals for the Fifth Circuit.
• In March 2014, the Company received a subpoena from the United States Attorney’s Office for the District of
Rhode Island, requesting documents and information concerning bona fide service fees and rebates received from
pharmaceutical manufacturers in connection with certain drugs utilized under Part D of the Medicare Program, as
well as the reporting of those fees and rebates to Part D plan sponsors. The Company has been cooperating with
the government and providing documents and information in response to the subpoena.
• The U.S. Department of Justice, through the U.S. Attorney’s Office for the Western District of Virginia, investigated
whether Omnicare’s activities in connection with the agreements it had with the manufacturer of the pharmaceutical
Depakote violated the False Claims Act or the Anti-Kickback Statute. Omnicare cooperated with this investigation
and believes that it has complied with applicable laws and regulations with respect to this matter. In connection with
this matter, on December 22, 2014, the U.S. Department of Justice filed a civil complaint-in-intervention in two
qui
tam
complaints, entitled United States,
et al.
,
ex rel
. Spetter v. Abbott Laboratories, Inc., Omnicare, Inc., and
PharMerica Corp., No. 1:07-cv-00006 and United States,
et al., ex rel.
McCoyd v. Abbott Laboratories, Omnicare,
Inc., PharMerica Corp., and Miles White, No. 1:07-cv-00081, alleging civil violations of the False Claims Act in
connection with the manufacturer agreements described above. In July 2015, the parties filed a Joint Motion to
Stay the Litigation stating that the parties have reached a proposed resolution of the monetary terms of a potential
settlement agreement. These financial terms are contingent on approval by authorized officials at the Department
of Justice, negotiation of terms of a settlement agreement, approval and releases from the OIG, the National
Association of Medicaid Fraud Control Units, and the Department of Justice, and coordination with discussions
with the United States regarding other ongoing matters. While the Company believes that a final settlement will be
reached, there can be no assurance that any final settlement agreement will be reached or as to the final terms of
such settlement.
• In May 2015, the Company entered into a settlement agreement with the U.S. Attorney’s Office for the Middle
District of Florida, resolving alleged violations of the Controlled Substances Act (“CSA”). The Company paid a
fine of $22 million in connection with the settlement. The Company is also undergoing several audits by the Drug
Enforcement Agency (“DEA”) Administrator and is in discussions with the DEA and the U.S. Attorney’s Office in
several locations concerning allegations that the Company has violated certain requirements of the CSA. Whether
agreements can be reached and on what terms is uncertain.
• In May 2015, the Company received a subpoena from the OIG requesting information and documents concerning
the Company’s automatic refill programs, adherence outreach programs, and pharmacy customer incentives,
particularly in connection with claims for reimbursement made to the Minnesota Medicaid program. The Company
has been cooperating with the investigation and providing information in response to the subpoena.
• In July 2015, the U.S. District Court in the District of Massachusetts dismissed all claims alleged in a
qui tam
lawsuit that had been brought against the Company by a pharmacy auditor and a former CVS pharmacist. The
lawsuit, which was initially filed under seal in 2011, alleged that the Company violated the federal False Claims
Act, as well as the false claims acts of several states, by overcharging state and federal governments in connec-
tion with prescription drugs available through the Company’s Health Savings Pass program, a membership-based
program that allows enrolled customers special pricing for typical 90-day supplies of various generic prescription
drugs. The federal government had declined to intervene in the case. The plaintiffs are appealing the dismissal to
the U.S. Court of Appeals for First Circuit.
• On July 27, 2015, a consolidated class action complaint was filed by plaintiffs naming Omnicare, the members
of the Omnicare Board of Directors, CVS Health, CVS Pharmacy, Inc. and its merger subsidiary as defendants.
The complaint alleged that the members of the Omnicare Board of Directors breached their fiduciary duties to
Omnicare’s stockholders during merger negotiations by entering into the merger agreement and approving the
merger, and the CVS parties aided and abetted such breaches of fiduciary duties. In September 2015, the court
granted plaintiffs’ voluntary notice of dismissal of all allegations against the defendants.