Quest Diagnostics 2003 Annual Report Download - page 9
Download and view the complete annual report
Please find page 9 of the 2003 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
7
WHAT MATTERS M OST...
When a doctor orders laboratory testing for you or a
loved one, quality is the only thing that really matters.
We dedicate time, effort, and resources to ensure the
highest quality in everything we do – to earn the trust
of our physicians and patients.
At Quest Diagnostics, we strive for perfection. Our
goal is to ensure highly appropriate patient care by
continually improving the quality of the processes
we use inside and outside the laboratory. We focus on
every aspect of what we do.
We use a methodology called Six Sigma that
provides the framework for setting performance
goals based on customer requirements. It also
helps us measure results and identify ways to
continually improve the processes we use in every
part of the business. Our more than 37,000
employees have been taught the basics of Six Sigma.
Nearly 1,000 employees have undergone more
rigorous training and lead quality improvement
projects. We have completed more than 1,000
projects that have improved quality in our operations,
from speeding the time it takes to provide a doctor
with test results, to reducing wait times at patient
service centers, to improving the accuracy of our
billing process.
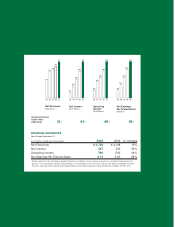
RIGOROUS QUALITY ASSURANCE
Clinical laboratories are subject to stringent
regulations imposed by federal, state and local
government agencies. All Quest Diagnostics
laboratories are accredited by the College of American
Pathologists (CAP), an independent non-governmental
organization of board-certified pathologists.
Additionally, Quest Diagnostics’ own internal quality
assurance processes exceed the standards set by
accrediting agencies, and include periodic random
reviews of completed anatomic pathology cases
and clinical pathology assays.
We invest to ensure that we have highly trained
and skilled people, and we support the continuing
education of our medical professionals to enable
them to keep up with the latest advances in diagnostic
anatomic pathology and clinical pathology.
Striving to provide the highest quality helps to clearly
distinguish us from the competition.
From Top:
Jessie Lee, M.D.
Associate Director,
Dermatopathology
Brian Geschwindt
Associate Master Black Belt,
Six Sigma
Vasundhara Untawale, M.D.
Associate Director,
Urologic Pathology