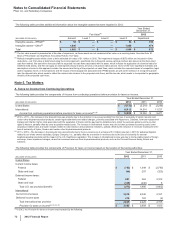
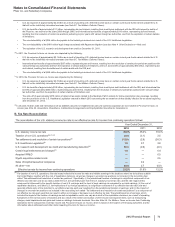
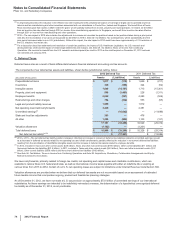
Pfizer 2012 Annual Report Download - page 72
Download and view the complete annual report
Please find page 72 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
2012 Financial Report
71
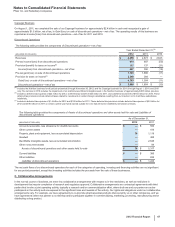
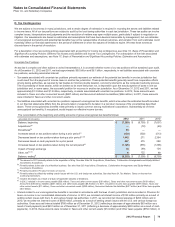
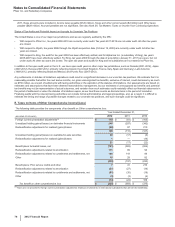
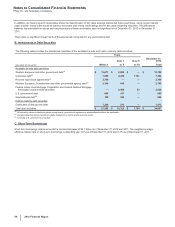
Note 4. Other Deductions—Net
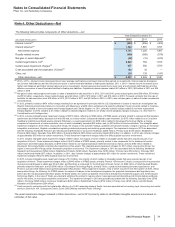
The following table provides components of Other deductions––net:
Year Ended December 31,
(MILLIONS OF DOLLARS) 2012 2011 2010
Interest income(a) $(383)$(456)$ (400)
Interest expense(a) 1,524 1,681 1,797
Net interest expense 1,141 1,225 1,397
Royalty-related income (469)(569)(579)
Net gain on asset disposals(b) (52)(15)(243)
Certain legal matters, net(c) 2,220 784 1,723
Certain asset impairment charges(d) 927 902 1,790
Costs associated with the separation of Zoetis(e) 125 33 —
Other, net 139 139 (147)
Other deductions––net $4,031 $2,499 $3,941
(a) 2012 v. 2011––Interest income decreased due to lower average cash balances and lower interest rates earned on investments. Interest expense decreased
due to lower debt balances and the effective conversion of some fixed-rate liabilities to floating-rate liabilities. 2011 v. 2010––Interest income increased due to
higher cash balances and higher interest rates earned on investments. Interest expense decreased due to lower long- and short-term debt balances and the
effective conversion of some fixed-rate liabilities to floating rate liabilities. Capitalized interest expense totaled $41 million in 2012, $50 million in 2011 and $36
million in 2010.
(b) Net gains include realized gains and losses on sales of available-for-sale securities: in 2012, 2011 and 2010, gross realized gains were $39 million, $79 million
and $153 million, respectively. Gross realized losses were $6 million in 2012, $73 million in 2011 and $12 million in 2010. Proceeds, primarily from the sale of
available-for-sale securities, were $19 billion in 2012, $10.2 billion in 2011 and $5.3 billion in 2010. In 2010, also includes gains on sales of certain investments
and businesses.
(c) In 2012, primarily includes a $491 million charge resulting from an agreement-in-principle with the U.S. Department of Justice to resolve an investigation into
Wyeth's historical promotional practices in connection with Rapamune, a $450 million settlement of a lawsuit by Brigham Young University related to Celebrex,
and charges related to hormone-replacement therapy litigation and Chantix litigation. In 2011, primarily includes charges related to hormone-replacement
therapy litigation. In 2010, includes a $1.3 billion charge for asbestos litigation related to our wholly owned subsidiary, Quigley Company, Inc. (See Note 17.
Commitments and Contingencies.)
(d) In 2012, includes intangible asset impairment charges of $872 million, reflecting (i) $393 million of IPR&D assets, primarily related to compounds that targeted
autoimmune and inflammatory diseases (full write-off) and, to a lesser extent, compounds related to pain treatment; (ii) $175 million related to our Consumer
Healthcare indefinite-lived brand assets, primarily Robitussin, a cough suppressant; (iii) $279 million related to Developed Technology Rights, a charge
comprised of impairments of various products, none of which individually exceeded $45 million; and (iv) $25 million of finite-lived brands. The intangible asset
impairment charges for 2012 reflect, among other things, the impact of new scientific findings, updated commercial forecasts, changes in pricing, an increased
competitive environment, litigation uncertainties regarding intellectual property and declining gross margins. The impairment charges in 2012 are associated
with the following: Worldwide Research and Development ($303 million); Consumer Healthcare ($200 million); Primary Care ($135 million); Established
Products ($83 million); Specialty Care ($56 million); Emerging Markets ($56 million) and Animal Health ($39 million). In addition, in 2012, also includes charges
of approximately $55 million for certain investments. These investment impairment charges reflect the difficult global economic environment.
In 2011, includes intangible asset impairment charges of $851 million, the majority of which relates to intangible assets that were acquired as part of our
acquisition of Wyeth. These impairment charges reflect (i) $475 million of IPR&D assets, primarily related to two compounds for the treatment of certain
autoimmune and inflammatory diseases; (ii) $193 million related to our biopharmaceutical indefinite-lived brand, Xanax; and (iii) $183 million related to
Developed Technology Rights comprising the impairment of five assets. The intangible asset impairment charges for 2011 reflect, among other things, the
impact of new scientific findings and an increased competitive environment. The impairment charges in 2011 are associated with the following: Worldwide
Research and Development ($394 million); Established Products ($193 million); Specialty Care ($135 million); Primary Care ($56 million); Oncology ($56
million) and Animal Health ($17 million). In addition, in 2011, also includes charges of approximately $51 million for certain investments. These investment
impairment charges reflect the difficult global economic environment.
In 2010, includes intangible asset impairment charges of $1.8 billion, the majority of which relates to intangible assets that were acquired as part of our
acquisition of Wyeth. These impairment charges reflect (i) $945 million of IPR&D assets, primarily Prevnar 13/Prevenar 13 Adult, a compound for the prevention
of pneumococcal disease in adults age 50 and older, and Neratinib, a compound for the treatment of breast cancer; (ii) $292 million of indefinite-lived Brands,
primarily related to Robitussin; and (iii) $540 million of Developed Technology Rights, primarily Thelin, a product that treated pulmonary hypertension, and
Protonix, a product that treats erosive gastroesophageal reflux disease. These impairment charges, most of which occurred in the third quarter of 2010, reflect,
among other things, the following: for IPR&D assets, the impact of changes to the development programs, the projected development and regulatory time-
frames and the risk associated with these assets; for Brand assets, the current competitive environment and planned investment support; and, for Developed
Technology Rights, in the case of Thelin, we voluntarily withdrew the product in regions where it was approved and discontinued all clinical studies worldwide,
and for the others, an increased competitive environment. The impairment charges in 2010 are generally associated with the following: Specialty Care ($708
million); Oncology ($396 million); Consumer Healthcare ($292 million); Established Products ($182 million); Primary Care ($145 million); and Worldwide
Research and Development ($54 million).
(e) Costs incurred in connection with the initial public offering of a 19.8% ownership stake in Zoetis. Includes expenditures for banking, legal, accounting and similar
services. (See Note 19A. Subsequent Events: Zoetis Debt Offering and Initial Public Offering.)
The asset impairment charges included in Other deductions––net in 2012 primarily relate to identifiable intangible assets and are based on
estimates of fair value.