Pfizer 2012 Annual Report Download - page 108
Download and view the complete annual report
Please find page 108 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
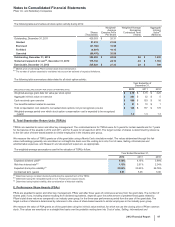
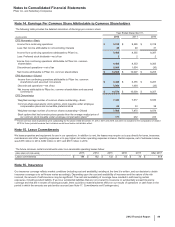
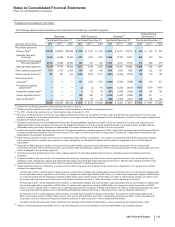
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
2012 Financial Report
107
patents relating to Neurontin, as well as engaging in off-label marketing of Neurontin. Plaintiffs seek compensatory damages on behalf of the
class, which may be subject to trebling.
Lipitor
•Whistleblower Action
In 2004, a former employee filed a “whistleblower” action against us in the U.S. District Court for the Eastern District of New York. The
complaint remained under seal until September 2007, at which time the U.S. Attorney for the Eastern District of New York declined to intervene
in the case. We were served with the complaint in December 2007. Plaintiff alleges off-label promotion of Lipitor in violation of the Federal Civil
False Claims Act and the false claims acts of certain states, and he seeks treble damages and civil penalties on behalf of the federal
government and the specified states as the result of their purchase, or reimbursement of patients for the purchase, of Lipitor allegedly for such
off-label uses. Plaintiff also seeks compensation as a whistleblower under those federal and state statutes. In addition, plaintiff alleges that he
was wrongfully terminated, in violation of the anti-retaliation provisions of applicable federal and New York law, and he seeks damages and the
reinstatement of his employment. In 2009, the court dismissed without prejudice the off-label promotion claims and, in 2010, plaintiff filed an
amended complaint containing off-label promotion allegations that are substantially similar to the allegations in the original complaint. In
November 2012, the District Court dismissed the amended complaint. In December 2012, the plaintiff appealed the District Court's decision to
the U.S. Court of Appeals for the Second Circuit.
•Antitrust Actions
Beginning in November 2011, purported class actions relating to Lipitor were filed in various federal courts against Pfizer, certain affiliates of
Pfizer, and, in most of the actions, Ranbaxy, among others. The plaintiffs in these various actions seek to represent nationwide, multi-state or
statewide classes consisting of persons or entities who directly purchased, indirectly purchased or reimbursed patients for the purchase
of Lipitor (or, in certain of the actions, generic Lipitor) from any of the defendants from March 2010 until the cessation of the defendants’
allegedly unlawful conduct (the Class Period). The plaintiffs allege delay in the launch of generic Lipitor, in violation of federal antitrust laws
and/or state antitrust, consumer protection and various other laws, resulting from (i) the 2008 agreement pursuant to which Pfizer and
Ranbaxy settled certain patent litigation involving Lipitor, and Pfizer granted Ranbaxy a license to sell a generic version of Lipitor in various
markets beginning on varying dates, and (ii) in certain of the actions, the procurement and/or enforcement of certain patents for Lipitor. Each
of the actions seeks, among other things, treble damages on behalf of the putative class for alleged price overcharges for Lipitor (or, in certain
of the actions, generic Lipitor) during the Class Period. In addition, individual actions have been filed against Pfizer, Ranbaxy and certain of
their affiliates, among others, that assert claims and seek relief for the plaintiffs that are substantially similar to the claims asserted and the
relief sought in the purported class actions described above. These various actions have been consolidated for pre-trial proceedings in a Multi-
District Litigation (In re Lipitor Antitrust Litigation MDL-2332) in the U.S. District Court for the District of New Jersey.
Chantix/Champix
•Actions in the U.S.
A number of individual lawsuits have been filed against us in various federal and state courts alleging suicide, attempted suicide and other
personal injuries as a result of the purported ingestion of Chantix, as well as economic loss. Plaintiffs in these actions seek compensatory and
punitive damages and the disgorgement of profits resulting from the sale of Chantix. In October 2009, the federal cases were transferred for
consolidated pre-trial proceedings to a Multi-District Litigation (In re Chantix (Varenicline) Products Liability Litigation MDL-2092) in the U.S.
District Court for the Northern District of Alabama.
In late-November 2012, we began advanced settlement discussions with various law firms that represent the plaintiffs in the majority of these
actions as well as persons who have asserted claims but not filed legal actions. As of February 2013, we had settled, or entered into definitive
agreements or agreements-in-principle to settle, approximately 80% of the known Chantix claims in the U.S., including actions pending in the
MDL and in state courts. In connection with these settlements and settlement agreements and agreements-in-principle, we recorded
aggregate charges in 2012 of approximately $273 million. In addition, we recorded aggregate charges in 2012 of approximately $15 million
that provide for the expected costs to resolve all remaining Chantix actions in the MDL and in state courts and all other known Chantix claims
in the U.S. The approximately $15 million aggregate charges are an estimate, and while we cannot estimate the range of reasonably possible
loss in excess of the amounts accrued given the uncertainties inherent in this litigation, as described below, additional charges may be
required in the future in connection with certain pending actions and claims and unknown claims relating to Chantix.
The federal Chantix actions were consolidated in the MDL more than three years ago, and the unresolved Chantix federal and state actions
and other known, unresolved Chantix claims could take many more years to resolve. However, opportunistic settlements could occur at any
time. The litigation process is time-consuming, as every Chantix action being litigated involves contested issues of medical causation and
knowledge of risk. Although the vast majority of Chantix actions allege neuropsychiatric injuries, the nature of the alleged injuries varies widely,
from completed suicide to attempted suicide resulting in hospitalization to the exacerbation of pre-existing depression or anxiety. In addition to
the widely varying types of injuries at issue, the underlying facts (e.g., medical causation; smoking, psychiatric and family history; reliance on
warnings; physician/patient interaction; analysis of labels; actual, provable injury; and other critical factors) can differ significantly from action
to action, and the process of discovery has not yet begun for a majority of the unresolved actions. In addition, the Chantix litigation involves
fundamental issues of science and medicine that often are uncertain and continue to evolve. As a result of the foregoing factors, we are
unable to estimate the range of reasonably possible loss in excess of the amounts accrued.