Pfizer 2012 Annual Report Download - page 103
Download and view the complete annual report
Please find page 103 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
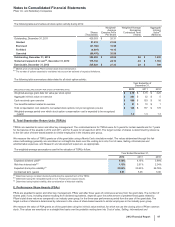
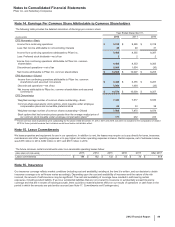
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
102
2012 Financial Report
their generic versions of Protonix tablets at risk in December 2007 and January 2008, respectively. Wyeth launched its own generic version of
Protonix tablets in January 2008, and Wyeth and Nycomed filed amended complaints in the pending patent-infringement action seeking
compensation for damages resulting from Teva USA’s, Teva Pharmaceutical Industries’ and Sun's at-risk launches.
In April 2010, the jury in the pending patent-infringement action upheld the validity of the basic patent for Protonix. In July 2010, the court
upheld the jury verdict, but it did not issue a judgment against Teva USA, Teva Pharmaceutical Industries or Sun because of their other claims
relating to the patent that still are pending. Wyeth and Nycomed will continue to pursue all available legal remedies against those generic
manufacturers, including compensation for damages resulting from their at-risk launches.
Separately, Wyeth and Nycomed are defendants in purported class actions brought by direct and indirect purchasers of Protonix in the U.S.
District Court for the District of New Jersey. Plaintiffs seek damages, on behalf of the respective putative classes, for the alleged violation of
antitrust laws in connection with the procurement and enforcement of the patents for Protonix. These purported class actions have been
stayed pending resolution of the underlying patent litigation in the U.S. District Court for the District of New Jersey.
Rapamune (sirolimus)
In March 2010, Watson Laboratories Inc. - Florida (Watson Florida) notified us that it had filed an abbreviated new drug application with the
FDA seeking approval to market a generic version of Rapamune. Watson Florida asserted the invalidity and non-infringement of a method-of-
use patent which (including the six-month pediatric exclusivity period) expires in January 2014 and a solid-dosage formulation patent which
(including the six-month pediatric exclusivity period) expires in 2018. In April 2010, we filed actions against Watson Florida and three other
Watson entities in the U.S. District Courts for the District of Delaware and the Southern District of Florida asserting the infringement of the
method-of-use patent. In June 2010, our action in the Southern District of Florida was transferred to the District of Delaware and consolidated
with our pending action there. In January 2013, the court ruled that the method-of-use patent is valid and infringed, thereby preventing Watson
Florida and the three other Watson entities from marketing a generic version of Rapamune in the U.S. prior to the expiration of that patent,
subject to a possible appeal of the decision by Watson Florida and the three other Watson entities.
Tygacil (tigecycline)
In October 2009, Sandoz, Inc., a division of Novartis AG (Sandoz), notified Wyeth that it had filed an abbreviated new drug application with the
FDA seeking approval to market a generic version of Tygacil. Sandoz asserts the invalidity and non-infringement of two of Wyeth’s patents
relating to Tygacil, including the basic patent, which expires in 2016. In December 2009, Wyeth filed suit against Sandoz in the U.S. District
Court for the District of Delaware asserting infringement of the basic patent. In January 2013, this action was settled on terms that are not
material to Pfizer.
EpiPen
King Pharmaceuticals, Inc. (King) brought a patent-infringement action against Sandoz in the U.S. District Court for the District of New Jersey
in July 2010 as the result of its abbreviated new drug application with the FDA seeking approval to market an epinephrine injectable product.
Sandoz is challenging patents, which expire in 2025, covering the next-generation autoinjector for use with epinephrine that is sold under the
EpiPen brand name.
Embeda (morphine sulfate/naltrexone hydrochloride extended-release capsules)
In August 2011, Watson Florida notified us that it had filed an abbreviated new drug application with the FDA seeking approval to market a
generic version of Embeda extended-release capsules. Watson Florida asserts the invalidity and non-infringement of three formulation patents
that expire in 2027. In October 2011, we filed an action against Watson Florida in the U.S. District Court for the District of Delaware asserting
the infringement of, and defending against the allegations of the invalidity of, the three formulation patents.
Torisel (temsirolimus)
In December 2011, we brought patent-infringement actions in the U.S. District Court for the District of Delaware against Sandoz and Accord
Healthcare, Inc. USA and certain of its affiliates (collectively, Accord) as a result of their abbreviated new drug applications with the FDA
seeking approval to market generic versions of Torisel before the expiration of the basic patent in 2014. In May 2012, we brought an action in
the same court against Sandoz for infringement of a formulation patent that expires in 2026. In September 2012, our actions against Sandoz
and Accord were consolidated in the District of Delaware.
Pristiq (desvenlafaxine)
Beginning in May 2012, several generic manufacturers notified us that they had filed abbreviated new drug applications with the FDA seeking
approval to market generic versions of Pristiq. Each of the generic manufacturers asserts the invalidity, unenforceability and/or non-
infringement of one or both of two patents for Pristiq that expire in 2022 and in 2027. Beginning in June 2012, we filed actions against these
generic manufacturers in the U.S. District Court for the District of Delaware and, in certain instances, also in other jurisdictions asserting the
validity, enforceability and infringement of those patents.
ACTION IN WHICH WE WERE THE DEFENDANT
Lipitor (atorvastatin)
In the U.K., while the patent protection for Lipitor expired in November 2011, the exclusivity period was extended by six months to May 6, 2012
by virtue of the pediatric extension to the supplementary protection certificate. In September 2011, Dr. Reddy’s Laboratories (U.K.) Limited
filed an action in the High Court of Justice seeking revocation of the six-month pediatric extension and damages resulting from the inability to
launch its generic Lipitor product during the pediatric extension period in the U.K. and certain other European Union (EU) markets. The action
was based upon the interpretation of the EU Pediatric Medicines Regulation. In December 2012, the court decided in our favor, rejecting Dr.
Reddy's claim in its entirety. In January 2013, this action was settled on terms that are not material to Pfizer.