Pfizer 2012 Annual Report Download - page 17
Download and view the complete annual report
Please find page 17 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
16
2012 Financial Report
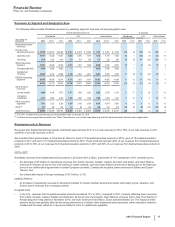
Revenues exceeded $500 million in each of 16 countries outside the U.S. in 2012 and 2011, and in each of 17 countries outside the U.S. in
2010. The U.S. and Japan were the only countries to contribute more than 10% of total revenue in 2012. The U.S. was the only country to
contribute more than 10% of total revenues in 2011 and 2010.
Our policy relating to the supply of pharmaceutical inventory at domestic wholesalers, and in major international markets, is to generally
maintain stocking levels under one month on average and to keep monthly levels consistent from year to year based on patterns of utilization.
We historically have been able to closely monitor these customer stocking levels by purchasing information from our customers directly or by
obtaining other third-party information. We believe our data sources to be directionally reliable but cannot verify their accuracy. Further, as we
do not control this third-party data, we cannot be assured of continuing access. Unusual buying patterns and utilization are promptly
investigated.
As is typical in the biopharmaceutical industry, our gross product sales are subject to a variety of deductions, that generally are estimated and
recorded in the same period that the revenues are recognized and primarily represent rebates and discounts to government agencies,
wholesalers, distributors and managed care organizations with respect to our pharmaceutical products. These deductions represent estimates
of the related obligations and, as such, judgment and knowledge of market conditions and practice are required when estimating the impact of
these sales deductions on gross sales for a reporting period. Historically, our adjustments to actual results have not been material to our
overall business. On a quarterly basis, our adjustments to actual results generally have been less than 1% of biopharmaceutical net sales and
can result in either a net increase or a net decrease in income. Product-specific rebate charges, however, can have a significant impact on
year-over-year individual product growth trends.
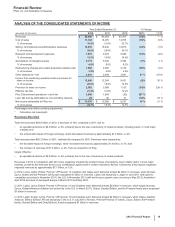
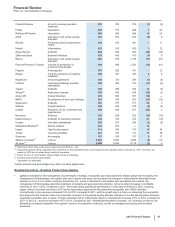
The following table provides information about certain deductions from revenues:
Year Ended December 31,
(BILLIONS OF DOLLARS) 2012 2011 2010
Medicaid and related state program rebates(a) $0.9$ 1.2 $ 1.3
Medicare rebates(a) 0.7 1.4 1.3
Performance-based contract rebates(a), (b) 2.2 3.5 2.6
Chargebacks(c) 3.6 3.2 3.0
Sales allowances(d) 4.7 4.9 4.5
Total $12.1 $14.2 $12.7
(a) Rebates are product-specific and, therefore, for any given year are impacted by the mix of products sold.
(b) Performance-based contract rebates include contract rebates with managed care customers within the U.S., including health maintenance organizations and
pharmacy benefit managers, who receive rebates based on the achievement of contracted performance terms and claims under these contracts.
(c) Chargebacks primarily represent reimbursements to wholesalers for honoring contracted prices to third parties.
(d) Sales allowances primarily represent pharmaceutical rebates, discounts and price reductions that are contractual or legislatively mandated outside the U.S.
The total rebates, chargebacks and sales allowances for 2012 were lower than 2011, primarily as a result of:
• the impact of decreased Medicaid, Medicare and performance-based contract rebates contracted for Lipitor and certain other
products that have lost exclusivity;
• changes in product mix; and
• the impact on chargebacks of decreased sales for certain products that have lost exclusivity,
partially offset by, among other factors:
• an increase in chargebacks for our branded products as a result of increasing competitive pressures.
Our accruals for Medicaid rebates, Medicare rebates, performance-based contract rebates, sales allowances and chargebacks were $3.8
billion as of December 31, 2012 and $4.8 billion as of December 31, 2011, and substantially all are included in Other current liabilities in our
Consolidated Balance Sheets.