Pfizer 2012 Annual Report Download - page 28
Download and view the complete annual report
Please find page 28 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
2012 Financial Report
27
(f) Two “approvable” letters were received by Wyeth in April and December 2007 from the FDA for Viviant (bazedoxifene), for the prevention of post-menopausal
osteoporosis, that set forth the additional requirements for approval. In May 2008, Wyeth received an “approvable” letter from the FDA for the treatment of post-
menopausal osteoporosis. The FDA is seeking additional data, and we have been systematically working through these requirements and seeking to address
the FDA's concerns. A full response will be provided to the FDA. In February 2008, the FDA advised Wyeth that it expects to convene an advisory committee to
review the pending NDAs for both the treatment and prevention indications after we submit our response to the “approvable” letters. In April 2009, Wyeth
received approval in the EU for CONBRIZA (the EU trade name for Viviant) for the treatment of post-menopausal osteoporosis in women at increased risk of
fracture. Viviant was also approved in Japan in July 2010 for the treatment of post-menopausal osteoporosis and in South Korea in November 2011 for the
treatment and prevention of post-menopausal osteoporosis.
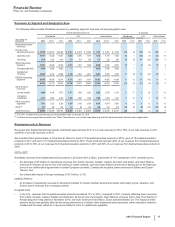
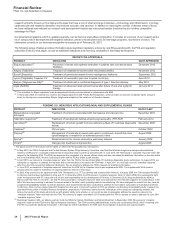
REGULATORY APPROVALS AND FILINGS IN THE EU AND JAPAN
PRODUCT DESCRIPTION OF EVENT
DATE
APPROVED DATE FILED*
Eliquis (Apixaban)(a) Approval in Japan for prevention of ischemic stroke and systemic
embolism in patients with nonvalvular atrial fibrillation
December 2012 —
Toviaz Approval in Japan for treatment of overactive bladder December 2012 —
Eliquis (Apixaban)(a) Approval in the EU for prevention of stroke and systemic
embolism in patients with nonvalvular atrial fibrillation
November 2012 —
Xalkori (Crizotinib) Conditional marketing authorization in the EU for treatment of
previously treated ALK-positive advanced non-small cell lung
cancer
October 2012 —
Inlyta (Axitinib) Approval in the EU for treatment of advanced renal cell
carcinoma after failure of prior systemic treatment
September 2012 —
Sutent Approval in Japan for treatment of pancreatic neuroendocrine
tumor
August 2012 —
Bazedoxifene-conjugated
estrogens
Application filed in the EU for treatment of symptoms associated
with menopause and osteoporosis
— July 2012
Prevenar 13 Infant Application filed in Japan for prevention of invasive
pneumococcal disease in infants and young children
— July 2012
Lyrica (Pregabalin) Approval in Japan for treatment of fibromyalgia June 2012 —
Inlyta (Axitinib) Approval in Japan for treatment of renal cell carcinoma not
indicated for curative resection, metastatic renal cell carcinoma
June 2012 —
Xalkori (Crizotinib) Approval in Japan for treatment of ALK-positive advanced non-
small cell lung cancer
March 2012 —
Lyrica (Pregabalin) Application filed in Japan for treatment of neuropathic pain:
peripheral neuropathic pain, central neuropathic pain
— March 2012
Tofacitinib Application filed in Japan for treatment of rheumatoid arthritis — December 2011
Tofacitinib Application filed in the EU for treatment of moderate-to-severe
active rheumatoid arthritis
— November 2011
Bosutinib(b) Application filed in the EU for treatment of previously treated
chronic myelogenous leukemia
— August 2011
* For applications in the EU, the dates set forth in this column are the dates on which the European Medicines Agency (EMA) validated our submissions.
(a) This indication for Eliquis (apixaban) was developed and is being commercialized in collaboration with BMS.
(b) In January 2013, the EMA's Committee for Medicinal Products for Human Use (CHMP) issued an opinion recommending that bosutinib be granted conditional
approval for treatment of previously treated chronic myelogenous leukemia. The initial application was for the treatment of newly diagnosed chronic
myelogenous leukemia.
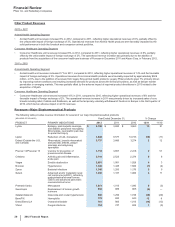
LATE-STAGE CLINICAL PROGRAMS FOR ADDITIONAL USES AND DOSAGE FORMS
FOR IN-LINE AND IN-REGISTRATION PRODUCTS
PRODUCT INDICATION
Eliquis (Apixaban) For the prevention and treatment of venous thromboembolism, which is being developed in collaboration
with BMS
Inlyta (Axitinib) Oral and selective inhibitor of vascular endothelial growth factor (VEGF) receptor 1, 2 & 3 for the
treatment of adjuvant renal cell carcinoma (Asia only)
Lyrica (Pregabalin) Peripheral neuropathic pain; CR (once-a-day) dosing
Sutent Adjuvant renal cell carcinoma
Tofacitinib A JAK kinase inhibitor for the treatment of psoriasis and ulcerative colitis
Xalkori (Crizotinib) An oral ALK and c-Met inhibitor for the treatment of ALK-positive 1st and 2nd line (supports potential full
approval in the U.S.) non-small cell lung cancer
Zithromax/chloroquine Malaria