Pfizer 2012 Annual Report Download - page 105
Download and view the complete annual report
Please find page 105 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
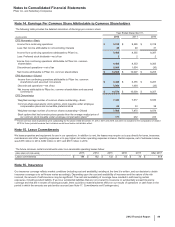
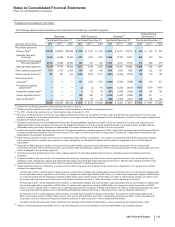
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
104
2012 Financial Report
alleged asbestos-related personal injury from exposure to Quigley products based on the “apparent manufacturer” theory of liability under
Pennsylvania law. The Second Circuit’s decision is procedural and does not address the merits of the plaintiffs’ claims under Pennsylvania law.
After the Second Circuit denied our petition for a rehearing, in September 2012, we filed a petition for certiorari with the U.S. Supreme Court
seeking a reversal of the Second Circuit’s decision. In July 2012, the Second Circuit had granted a stay of its decision while the U.S. Supreme
Court considers our petition for certiorari.
In a separately negotiated transaction with an insurance company in August 2004, we agreed to a settlement related to certain insurance
coverage which provides for payments to an insurance proceeds trust established by Pfizer and Quigley over a ten-year period of amounts
totaling $405 million. Most of these insurance proceeds, as well as other payments from insurers that issued policies covering Pfizer and
Quigley, would be paid, following confirmation, to the Trust for the benefit of present unsettled and future claimants with claims arising from
exposure to Quigley products.
•Other Matters
Between 1967 and 1982, Warner-Lambert owned American Optical Corporation, which manufactured and sold respiratory protective devices
and asbestos safety clothing. In connection with the sale of American Optical in 1982, Warner-Lambert agreed to indemnify the purchaser for
certain liabilities, including certain asbestos-related and other claims. As of December 31, 2012, approximately 66,400 claims naming
American Optical and numerous other defendants were pending in various federal and state courts seeking damages for alleged personal
injury from exposure to asbestos and other allegedly hazardous materials. Warner-Lambert is actively engaged in the defense of, and will
continue to explore various means to resolve, these claims.
Warner-Lambert and American Optical brought suit in state court in New Jersey against the insurance carriers that provided coverage for the
asbestos and other allegedly hazardous materials claims related to American Optical. A majority of the carriers subsequently agreed to pay for
a portion of the costs of defending and resolving those claims. The litigation continues against the carriers who have disputed coverage or how
costs should be allocated to their policies, and the court held that Warner-Lambert and American Optical are entitled to payment from each of
those carriers of a proportionate share of the costs associated with those claims. Under New Jersey law, a special allocation master was
appointed to implement certain aspects of the court’s rulings.
Numerous lawsuits are pending against Pfizer in various federal and state courts seeking damages for alleged personal injury from exposure
to products containing asbestos and other allegedly hazardous materials sold by Gibsonburg Lime Products Company (Gibsonburg).
Gibsonburg was acquired by Pfizer in the 1960s and sold products containing small amounts of asbestos until the early 1970s.
There also are a small number of lawsuits pending in various federal and state courts seeking damages for alleged exposure to asbestos in
facilities owned or formerly owned by Pfizer or its subsidiaries.
Celebrex and Bextra
Beginning in late 2004, actions, including purported class actions, were filed in various federal and state courts against Pfizer, Pharmacia
Corporation (Pharmacia) and certain current and former officers, directors and employees of Pfizer and Pharmacia. These actions include (i)
purported class actions alleging that Pfizer and certain current and former officers of Pfizer violated federal securities laws by misrepresenting
the safety of Celebrex and Bextra, and (ii) purported class actions filed by persons who claim to be participants in the Pfizer or Pharmacia
Savings Plan alleging that Pfizer and certain current and former officers, directors and employees of Pfizer or, where applicable, Pharmacia
and certain former officers, directors and employees of Pharmacia, violated certain provisions of the Employee Retirement Income Security
Act of 1974 (ERISA) by selecting and maintaining Pfizer stock or Pharmacia stock as an investment alternative when it allegedly no longer
was a suitable or prudent investment option. In June 2005, the federal securities and ERISA actions were transferred for consolidated pre-trial
proceedings to a Multi-District Litigation (In re Pfizer Inc. Securities, Derivative and "ERISA" Litigation MDL-1688) in the U.S. District Court for
the Southern District of New York. In the consolidated federal securities action in the Multi-District Litigation, the court in March 2012 certified a
class consisting of all persons who purchased or acquired Pfizer stock between October 31, 2000 and October 19, 2005. In November 2012,
several institutional investors that had opted out of the certified class filed three, separate, multi-plaintiff actions in the Southern District of New
York against the same defendants named in the consolidated class action, asserting allegations substantially similar to those asserted in the
consolidated class action.
Various Drugs: Off-Label Promotion Actions
In May 2010, a purported class action was filed in the U.S. District Court for the Southern District of New York against Pfizer and several of our
current and former officers. The complaint alleges that the defendants violated federal securities laws by making or causing Pfizer to make
false statements, and by failing to disclose or causing Pfizer to fail to disclose material information, concerning the alleged off-label promotion
of certain pharmaceutical products, alleged payments to physicians to promote the sale of those products and government investigations
related thereto. Plaintiffs seek damages in an unspecified amount. In March 2012, the court certified a class consisting of all persons who
purchased Pfizer common stock in the U.S. or on U.S. stock exchanges between January 19, 2006 and January 23, 2009 and were damaged
as a result of the decline in the price of Pfizer common stock allegedly attributable to the claimed violations.
Hormone-Replacement Therapy
•Personal Injury and Economic Loss Actions
Pfizer and certain wholly owned subsidiaries and limited liability companies, including Wyeth and King, along with several other
pharmaceutical manufacturers, have been named as defendants in approximately 10,000 actions in various federal and state courts alleging
personal injury or economic loss related to the use or purchase of certain estrogen and progestin medications prescribed for women to treat
the symptoms of menopause. Although new actions are occasionally filed, the number of new actions was not significant in the fourth quarter