Pfizer 2012 Annual Report Download - page 30
Download and view the complete annual report
Please find page 30 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
2012 Financial Report
29
• a shift in geographic and business mix; and
• the unfavorable impact of foreign exchange of 2% in 2011.
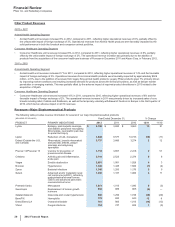
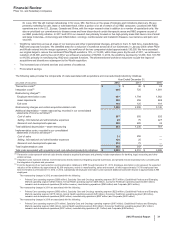
Selling, Informational and Administrative (SI&A) Expenses
Year Ended December 31, % Change
(MILLIONS OF DOLLARS) 2012 2011 2010 12/11 11/10
Selling, informational and administrative expenses $16,616 $18,832 $18,973 (12)(1)
2012 v. 2011
SI&A expenses decreased 12% in 2012, compared to 2011, primarily due to:
• savings generated from a reduction in the field force and a decrease in promotional spending, both partly in response to product losses of
exclusivity;
• more streamlined corporate support functions; and
• the favorable impact of foreign exchange of 2%,
partially offset by:
• costs associated with the separation of Zoetis employees, net assets and operations from Pfizer.
2011 v. 2010
SI&A expenses were largely unchanged in 2011, compared to 2010, primarily due to:
• the fee provided for under the U.S. Healthcare Legislation beginning in 2011;
• the addition of legacy King operating costs; and
• the unfavorable impact of foreign exchange of 2%,
offset by:
• savings associated with our cost-reduction and productivity initiatives.
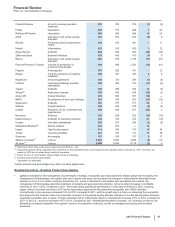
Research and Development (R&D) Expenses
Year Ended December 31, % Change
(MILLIONS OF DOLLARS) 2012 2011 2010 12/11 11/10
Research and development expenses $7,870 $9,074 $9,483 (13)(4)
2012 v. 2011
R&D expenses decreased 13% in 2012, compared to 2011, primarily due to:
• savings generated by the discontinuation of certain therapeutic areas and R&D programs in connection with our previously announced
cost-reduction and productivity initiatives; and
• lower charges related to implementing our cost-reduction and productivity initiatives,
partially offset by:
• a $250 million payment to AstraZeneca to obtain the exclusive global over-the-counter rights to Nexium.
2011 v. 2010
R&D expenses decreased 4% in 2011, compared to 2010, primarily due to:
• savings associated with our cost-reduction and productivity initiatives,
partially offset by:
• higher charges related to implementing our cost-reduction and productivity initiatives;
• the addition of legacy King expenses; and
• the unfavorable impact of foreign exchange of 1%.
R&D expenses also include payments for intellectual property rights of $371 million in 2012, $306 million in 2011 and $393 million in 2010 (for
further discussion, see the “Our Business Development Initiatives” section of this Financial Review).