Pfizer 2012 Annual Report Download - page 109
Download and view the complete annual report
Please find page 109 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
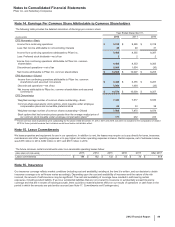
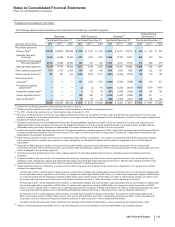
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
108
2012 Financial Report
•Actions in Canada
Beginning in December 2008, purported class actions were filed against us in the Ontario Superior Court of Justice (Toronto Region), the
Superior Court of Quebec (District of Montreal), the Court of Queen’s Bench of Alberta, Judicial District of Calgary, and the Superior Court of
British Columbia (Vancouver Registry) on behalf of all individuals and third-party payers in Canada who have purchased and ingested
Champix or reimbursed patients for the purchase of Champix. Each of these actions asserts claims under Canadian product liability law,
including with respect to the safety and efficacy of Champix, and, on behalf of the putative class, seeks monetary relief, including punitive
damages. In June 2012, the Ontario Superior Court of Justice certified the Ontario proceeding as a class action, defining the class as
consisting of the following: (i) all persons in Canada who ingested Champix during the period from April 2, 2007 to May 31, 2010 and who
experienced at least one of a number of specified neuropsychiatric adverse events; (ii) all persons who are entitled to assert claims in respect
of Champix pursuant to Canadian legislation as the result of their relationship with a class member; and (iii) all health insurers who are entitled
to assert claims in respect of Champix pursuant to Canadian legislation. The Ontario Superior Court of Justice certified the class against Pfizer
Canada Inc. only and ruled that the action against Pfizer Inc. should be stayed until after the trial of the issues that are common to the class
members. The actions in Quebec, Alberta and British Columbia have been stayed in favor of the Ontario action, which is proceeding on a
national basis.
Bapineuzumab
In June 2010, a purported class action was filed in the U.S. District Court for the District of New Jersey against Pfizer, as successor to Wyeth,
and several former officers of Wyeth. The complaint alleges that Wyeth and the individual defendants violated federal securities laws by
making or causing Wyeth to make false and misleading statements, and by failing to disclose or causing Wyeth to fail to disclose material
information, concerning the results of a clinical trial involving bapineuzumab, a product in development for the treatment of Alzheimer’s
disease. The plaintiff seeks to represent a class consisting of all persons who purchased Wyeth securities from May 21, 2007 through July
2008 and seeks damages in an unspecified amount on behalf of the putative class. In February 2012, the court granted the defendants’
motion to dismiss the complaint. In March 2012, the plaintiff filed a motion seeking the court’s permission to file an amended complaint. In
December 2012, the court granted the plaintiff's motion and, in January 2013, the defendants filed a motion to dismiss the amended complaint.
In July 2010, a related action was filed in the U.S. District Court for the Southern District of New York against Elan Corporation (Elan), certain
directors and officers of Elan, and Pfizer, as successor to Wyeth. Elan participated in the development of bapineuzumab until September
2009. The complaint alleges that Elan, Wyeth and the individual defendants violated federal securities laws by making or causing Elan to
make false and misleading statements, and by failing to disclose or causing Elan to fail to disclose material information, concerning the results
of a clinical trial involving bapineuzumab. The plaintiff seeks to represent a class consisting of all persons who purchased Elan call options
from June 17, 2008 through July 29, 2008 and seeks damages in an unspecified amount on behalf of the putative class. In June 2011, the
court granted Pfizer’s and Elan’s motions to dismiss the complaint. In July 2011, the plaintiff filed a supplemental memorandum setting forth
the bases that the plaintiff believed supported amendment of the complaint. In August 2011, the court dismissed the complaint with prejudice.
In February 2013, the U.S. Court of Appeals for the Second Circuit affirmed the District Court's dismissal of the complaint.
Thimerosal
Wyeth is a defendant in a number of suits by or on behalf of vaccine recipients alleging that exposure through vaccines to cumulative doses of
thimerosal, a preservative used in certain childhood vaccines formerly manufactured and distributed by Wyeth and other vaccine
manufacturers, caused severe neurological damage and/or autism in children. While several suits were filed as purported nationwide or
statewide class actions, all of the purported class actions have been dismissed, either by the courts or voluntarily by the plaintiffs. In addition
to the suits alleging injury from exposure to thimerosal, certain of the cases were brought by parents in their individual capacities for, among
other things, loss of services and loss of consortium of the injured child.
The National Childhood Vaccine Injury Act (the Vaccine Act) requires that persons alleging injury from childhood vaccines first file a petition in
the U.S. Court of Federal Claims asserting a vaccine-related injury. At the conclusion of that proceeding, petitioners may bring a lawsuit
against the manufacturer in federal or state court, provided that they have satisfied certain procedural requirements. Also under the terms of
the Vaccine Act, if a petition has not been adjudicated by the U.S. Court of Federal Claims within a specified time period after filing, the
petitioner may opt out of the proceeding and pursue a lawsuit against the manufacturer by following certain procedures. Some of the vaccine
recipients who have sued Wyeth to date may not have satisfied the conditions to filing a lawsuit that are mandated by the Vaccine Act. The
claims brought by parents for, among other things, loss of services and loss of consortium of the injured child are not covered by the Vaccine
Act.
In 2002, the Office of Special Masters of the U.S. Court of Federal Claims established an Omnibus Autism Proceeding with jurisdiction over
petitions in which vaccine recipients claim to suffer from autism or autism spectrum disorder as a result of receiving thimerosal-containing
childhood vaccines and/or the measles, mumps and rubella (MMR) vaccine. There currently are several thousand petitions pending in the
Omnibus Autism Proceeding. Special masters of the court have heard six test cases on petitioners’ theories that either thimerosal-containing
vaccines in combination with the MMR vaccine or thimerosal-containing vaccines alone can cause autism or autism spectrum disorder.
• In February 2009, special masters of the U.S. Court of Federal Claims rejected the three cases brought on the theory that a combination of
MMR and thimerosal-containing vaccines caused petitioners’ conditions. After these rulings were affirmed by the U.S. Court of Federal
Claims, two of them were appealed by petitioners to the U.S. Court of Appeals for the Federal Circuit. In 2010, the Federal Circuit affirmed
the decisions of the special masters in both of these cases.
• In March 2010, special masters of the U.S. Court of Federal Claims rejected the three additional test cases brought on the theory that
thimerosal-containing vaccines alone caused petitioners’ conditions. Petitioners did not seek review by the U.S. Court of Federal Claims of
the decisions of the special masters in these latter three test cases, and judgments were entered dismissing the cases in April 2010.
• Petitioners in each of the six test cases have filed an election to bring a civil action.