Pfizer 2012 Annual Report Download - page 110
Download and view the complete annual report
Please find page 110 of the 2012 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
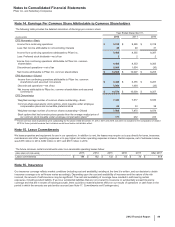
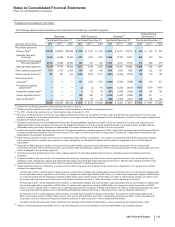
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
2012 Financial Report
109
Rebif
We have an exclusive collaboration agreement with EMD Serono, Inc. (Serono) to co-promote Rebif, a treatment for multiple sclerosis, in the
U.S. In August 2011, Serono filed a complaint in the Philadelphia Court of Common Pleas seeking a declaratory judgment that we are not
entitled to a 24-month extension of the Rebif co-promotion agreement, which otherwise would terminate at the end of 2013. We disagree with
Serono's interpretation of the agreement and believe that we have the right to extend the agreement to the end of 2015. In October 2011, the
court sustained our preliminary objections and dismissed Serono’s complaint, and Serono has appealed the decision to the Superior Court of
Pennsylvania.
Various Drugs: Co-Pay Programs
In March 2012, a purported class action was filed against Pfizer in the U.S. District Court for the Southern District of New York. The plaintiffs
seek to represent a class consisting of all entities in the U.S. and its territories that have reimbursed patients for the purchase of certain Pfizer
drugs for which co-pay programs exist or have existed. The plaintiffs allege that these programs violate the federal RICO Act and federal
antitrust law by, among other things, providing an incentive for patients to use certain Pfizer drugs rather than less-expensive competitor
products, thereby increasing the payers’ reimbursement costs. The plaintiffs seek treble damages on behalf of the putative class for their
excess reimbursement costs allegedly attributable to the co-pay programs as well as an injunction prohibiting us from offering such programs.
In July 2012, a substantially similar purported class action was filed against Pfizer in the U.S. District Court for the Southern District of Illinois,
which action was stayed in October 2012 pending the outcome of the action in the Southern District of New York. Similar purported class
actions have been filed against several other pharmaceutical companies.
A3. Legal Proceedings––Commercial and Other Matters
Average Wholesale Price Litigation
Pfizer, certain of its subsidiaries and other pharmaceutical manufacturers are defendants in actions in various state courts by a number of
states, as well as one purported class action by certain employee benefit plans and other third-party payers, alleging that the defendants
provided average wholesale price (AWP) information for certain of their products that was higher than the actual average prices at which those
products were sold. The AWP is used to determine reimbursement levels under Medicare Part B and Medicaid and in many private-sector
insurance policies and medical plans. The plaintiffs claim that the alleged spread between the AWPs at which purchasers were reimbursed
and the actual sale prices was promoted by the defendants as an incentive to purchase certain of their products. In addition to suing on their
own behalf, some of the plaintiff states seek to recover on behalf of individuals, private-sector insurance companies and medical plans in their
states. These various actions allege, among other things, fraud, unfair competition, unfair trade practices and the violation of consumer
protection statutes, and seek monetary and other relief, including civil penalties and treble damages.
Monsanto-Related Matters
In 1997, Monsanto Company (Former Monsanto) contributed certain chemical manufacturing operations and facilities to a newly formed
corporation, Solutia Inc. (Solutia), and spun off the shares of Solutia. In 2000, Former Monsanto merged with Pharmacia & Upjohn Company
to form Pharmacia Corporation (Pharmacia). Pharmacia then transferred its agricultural operations to a newly created subsidiary, named
Monsanto Company (New Monsanto), which it spun off in a two-stage process that was completed in 2002. Pharmacia was acquired by Pfizer
in 2003 and is now a wholly owned subsidiary of Pfizer.
In connection with its spin-off that was completed in 2002, New Monsanto assumed, and agreed to indemnify Pharmacia for, any liabilities
related to Pharmacia’s former agricultural business. New Monsanto is defending and indemnifying Pharmacia in connection with various
claims and litigation arising out of, or related to, the agricultural business.
In connection with its spin-off in 1997, Solutia assumed, and agreed to indemnify Pharmacia for, liabilities related to Former Monsanto's
chemical businesses. As the result of its reorganization under Chapter 11 of the U.S. Bankruptcy Code, Solutia’s indemnification obligations
related to Former Monsanto’s chemical businesses are limited to sites that Solutia has owned or operated. In addition, in connection with its
spinoff that was completed in 2002, New Monsanto assumed, and agreed to indemnify Pharmacia for, any liabilities primarily related to Former
Monsanto's chemical businesses, including, but not limited to, any such liabilities that Solutia assumed. Solutia's and New Monsanto's
assumption of and agreement to indemnify Pharmacia for these liabilities apply to pending actions and any future actions related to Former
Monsanto's chemical businesses in which Pharmacia is named as a defendant, including, without limitation, actions asserting environmental
claims, including alleged exposure to polychlorinated biphenyls. Solutia and New Monsanto are defending and indemnifying Pharmacia in
connection with various claims and litigation arising out of, or related to, Former Monsanto’s chemical businesses.
Trade Secrets Action in California
In 2004, Ischemia Research and Education Foundation (IREF) and its chief executive officer brought an action in California Superior Court,
Santa Clara County, against a former IREF employee and Pfizer. Plaintiffs allege that defendants conspired to misappropriate certain
information from IREF’s allegedly proprietary database in order to assist Pfizer in designing and executing a clinical study of a Pfizer drug. In
2008, the jury returned a verdict for compensatory damages of approximately $38.7 million. In March 2009, the court awarded prejudgment
interest, but declined to award punitive damages. In July 2009, the court granted our motion for a new trial and vacated the jury verdict. In
February 2013, the trial court's decision was affirmed by the California Court of Appeal, Sixth Appellate District.
Environmental Matters
In 2009, we submitted to the U.S. Environmental Protection Agency (EPA) a corrective measures study report with regard to Pharmacia
Corporation's discontinued industrial chemical facility in North Haven, Connecticut and a revised site-wide feasibility study with regard to
Wyeth’s discontinued industrial chemical facility in Bound Brook, New Jersey. In September 2010, our corrective measures study report with
regard to the North Haven facility was approved by the EPA, and we commenced construction of the site remedy in late 2011 under an
Updated Administrative Order on Consent with the EPA. In July 2011, we finalized an Administrative Settlement Agreement and Order on
Consent for Removal Action with the EPA with regard to the Bound Brook facility. In May 2012, we completed construction of an interim