Pfizer 2015 Annual Report Download - page 48
Download and view the complete annual report
Please find page 48 of the 2015 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
2015 Financial Report
47
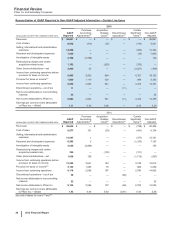
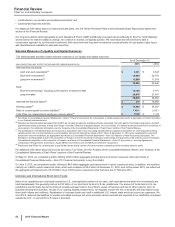
Global Established Pharmaceutical Operating Segment
2015 vs. 2014:
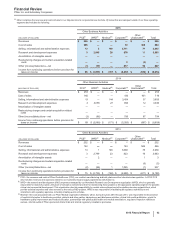
• Revenues decreased 14% in 2015, compared to 2014. Foreign exchange had an unfavorable impact of 7% on GEP revenues in 2015,
compared to 2014. Revenues decreased by 7% operationally in 2015, primarily due to the following operational factors:
the loss of exclusivity and associated launch of multi-source generic competition for Celebrex in the U.S. in December 2014, for Zyvox in
the U.S. beginning in the first half of 2015, for Lyrica in certain developed Europe markets beginning in the first quarter of 2015, and
Inspra in developed Europe markets beginning in August 2014 (collectively, down by approximately $2.5 billion in 2015);
a decline in Lipitor revenues in developed markets as a result of continued generic competition (down approximately $160 million in
2015);
the decline in Zosyn/Tazocin revenues due to a disruption in supply due to manufacturing issues (down approximately $160 million in
2015); and
the termination of the co-promotion collaboration for Spiriva (down approximately $110 million in 2015),
partially offset by:
the inclusion of legacy Hospira operations, which contributed $1.5 billion; and
growth in emerging markets (excluding legacy Hospira), where revenues increased 2% operationally in 2015 (up by approximately $160
million in 2015).
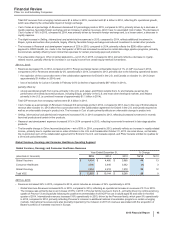
Total GEP revenues from emerging markets were $7.1 billion in 2015, compared to $7.5 billion in 2014, reflecting 3% operational growth,
which was more than offset by the unfavorable impact of foreign exchange of 9%.
• Cost of sales as a percentage of Revenues increased 2.6 percentage points in 2015, compared to 2014, primarily due to the impact of
losses of exclusivity resulting in an unfavorable change in product mix and the inclusion of legacy Hospira operations, partially offset by
favorable foreign exchange. The decrease in Cost of sales of 2% in 2015, compared to 2014, was primarily driven by favorable foreign
exchange and lower volumes as a result of products losing exclusivity, offset by the inclusion of legacy Hospira operations.
• Selling, informational and administrative expenses decreased 8% in 2015, compared to 2014, primarily due to lower field force, advertising
and promotional expenses reflecting the benefits of cost-reduction and productivity initiatives, as well as favorable foreign exchange,
partially offset by the inclusion of legacy Hospira operations, an increase in certain general and administrative expenses and higher cost for
the U.S. Branded Prescription Drug Fee compared to the prior year.
• Research and development expenses increased 15% in 2015, compared to 2014, reflecting the inclusion of legacy Hospira operations and
increased investment in biosimilar development programs and sterile injectable development programs acquired as part of our acquisition of
InnoPharma, Inc. partially offset by lower clinical trial expenses related to postmarketing commitments, primarily for Celebrex and Pristiq.
• The unfavorable change in Other (income)/deductions––net of 43% in 2015, compared to 2014, primarily reflects the non-recurrence of
prior year gains on the sale of product rights, unfavorable foreign exchange and a decrease in our equity income from our equity-method
investment in China (Hisun Pfizer), partially offset by other income gains.
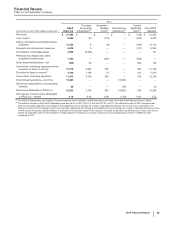
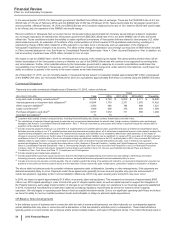
2014 vs. 2013:
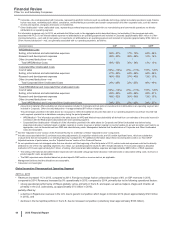
• Revenues decreased 9% in 2014, compared to 2013. Foreign exchange had an unfavorable impact of 2% on GEP revenues in 2014
compared to 2013. Revenues decrease 7% operationally in 2014, compared to 2013, primarily due to the following operational factors:
the loss of exclusivity and subsequent launch of multi-source generic competition for Detrol LA in the U.S. in January 2014, Celebrex in
the U.S. in December 2014 and developed Europe in November 2014, Viagra in most major European markets in June 2013 as well as
Aricept in Canada in December 2013 (aggregate decline of approximately $826 million in 2014);
the expiration or near-term expiration of the co-promotion collaboration for Spiriva in most countries, which has resulted in a decline in
Pfizer’s share of Spiriva revenues (down approximately $490 million in 2014);
a decline in branded Lipitor revenues in the U.S. and most other developed markets as a result of continued generic competition (down
approximately $388 million in 2014);
the decline of certain products, including Effexor, Norvasc, atorvastatin, Zosyn/Tazocin, Metaxalone, Ziprasidone and Tygacil (down
approximately $428 million in 2014);
a decline due to loss of exclusivity for certain other products in developed markets (down approximately $170 million in 2014); and
a decline in Aricept, not including Canada, revenues primarily due to the termination of the co-promotion agreement in Japan in
December 2012 (down approximately $75 million in 2014),
partially offset by:
the growth of Lipitor in China (up approximately $164 million in 2014);
the strong performance of Lyrica in Europe (growth of approximately $144 million in 2014); and
the contribution from the collaboration with Mylan Inc. to market generic drugs in Japan (approximately $37 million in 2014).
Total GEP revenues from emerging markets were $7.5 billion in 2014.