Pfizer 2015 Annual Report Download - page 44
Download and view the complete annual report
Please find page 44 of the 2015 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
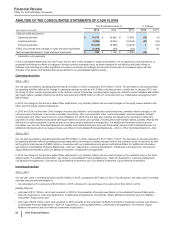
Financial Review
Pfizer Inc. and Subsidiary Companies
2015 Financial Report
43
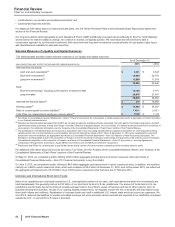
(c) Other comprises the revenues and costs included in our Adjusted income components (see footnote (d) below) that are managed outside of our three operating
segments and includes the following:
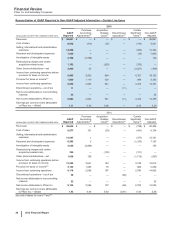
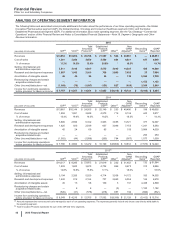
2015
Other Business Activities
(MILLIONS OF DOLLARS) PCS(i) WRD(ii) Medical(iii) Corporate(iv) Other
Unallocated(v) Total
Revenues $ 506 $ — $ — $ — $ — $ 506
Cost of sales 396 — — 20 468 884
Selling, informational and administrative expenses 13 2149 3,711 71 3,945
Research and development expenses 3 2,945 29 878 11 3,865
Amortization of intangible assets —— — — — —
Restructuring charges and certain acquisition-related
costs —— — 3 (3) —
Other (income)/deductions––net (1)(77) — 817 90 827
Income from continuing operations before provision for
taxes on income $96 $ (2,870) $ (177)$ (5,430)$ (636)$ (9,016)
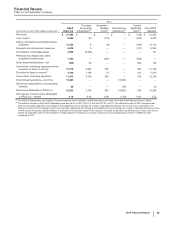
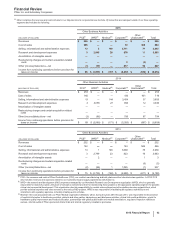
2014
Other Business Activities
(MILLIONS OF DOLLARS) PCS(i) WRD(ii) Medical(iii) Corporate(iv) Other
Unallocated(v) Total
Revenues $ 253 $ — $ — $ — $ — $ 253
Cost of sales 165 — — 100 451 716
Selling, informational and administrative expenses 19 —144 3,454 37 3,655
Research and development expenses 3 3,056 27 850 12 3,946
Amortization of intangible assets — — — — — —
Restructuring charges and certain acquisition-related
costs — — — — — —
Other (income)/deductions––net (3)(66) — 795 67 794
Income from continuing operations before provision for
taxes on income $ 69 $(2,989) $ (171)$ (5,200)$ (567)$ (8,859)
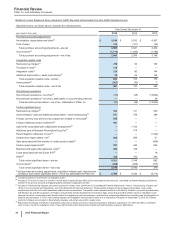
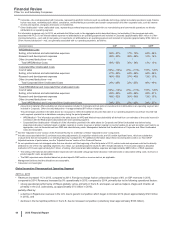
2013
Other Business Activities
(MILLIONS OF DOLLARS) PCS(i) WRD(ii) Medical(iii) Corporate(iv) Other
Unallocated(v) Total
Revenues $ 232 $ — $ — $ 1 $ — $ 232
Cost of sales 142 — — 143 582 866
Selling, informational and administrative expenses 14 1146 3,699 78 3,938
Research and development expenses 3 2,799 23 823 16 3,663
Amortization of intangible assets — 2 — — 13
Restructuring charges and certain acquisition-related
costs — — — — (5) (5)
Other (income)/deductions––net (2)(66) 1 1,025 (1) 957
Income from continuing operations before provision for
taxes on income $ 75 $ (2,735) $ (169)$ (5,689)$ (671)$ (9,189)
(i) PCS—the revenues and costs of Pfizer CentreSource (PCS), our contract manufacturing and bulk pharmaceutical chemical sales operation. In 2015, PCS
also includes revenues and expenses related to our manufacturing and supply agreements with Zoetis Inc.
(ii) WRD—the research and development (R&D) expenses managed by our Worldwide Research and Development organization (WRD), which is generally
responsible for research projects until proof-of-concept is achieved and then for transitioning those projects to the appropriate operating segment for possible
clinical and commercial development. This organization also has responsibility for certain science-based and other platform-services organizations, which
provide technical expertise and other services to the various R&D projects. WRD is also responsible for facilitating all regulatory submissions and
interactions with regulatory agencies, including all safety-event activities.
(iii) Medical—the costs associated with our Pfizer Medical organization (Medical), which, during the years 2013 through 2015, was responsible for the provision
of medical information to healthcare providers, patients and other parties, transparency and disclosure activities, clinical trial results publication, grants for
healthcare quality improvement and medical education, partnerships with global public health and medical associations, regulatory inspection readiness
reviews, internal audits of Pfizer-sponsored clinical trials and internal regulatory compliance processes.