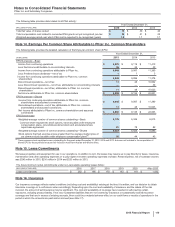
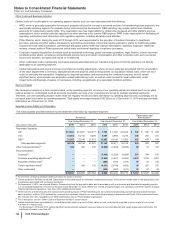
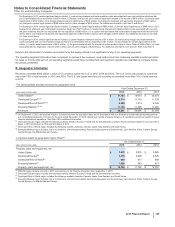
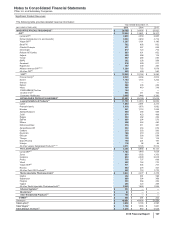
Pfizer 2015 Annual Report Download - page 122
Download and view the complete annual report
Please find page 122 of the 2015 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
2015 Financial Report
121
Monsanto-Related Matters
In 1997, Monsanto Company (Former Monsanto) contributed certain chemical manufacturing operations and facilities to a newly formed
corporation, Solutia Inc. (Solutia), and spun off the shares of Solutia. In 2000, Former Monsanto merged with Pharmacia & Upjohn Company
to form Pharmacia Corporation (Pharmacia). Pharmacia then transferred its agricultural operations to a newly created subsidiary, named
Monsanto Company (New Monsanto), which it spun off in a two-stage process that was completed in 2002. Pharmacia was acquired by Pfizer
in 2003 and is now a wholly owned subsidiary of Pfizer.
In connection with its spin-off that was completed in 2002, New Monsanto assumed, and agreed to indemnify Pharmacia for, any liabilities
related to Pharmacia’s former agricultural business. New Monsanto is defending and indemnifying Pharmacia in connection with various
claims and litigation arising out of, or related to, the agricultural business.
In connection with its spin-off in 1997, Solutia assumed, and agreed to indemnify Pharmacia for, liabilities related to Former Monsanto’s
chemical businesses. As the result of its reorganization under Chapter 11 of the U.S. Bankruptcy Code, Solutia’s indemnification obligations
relating to Former Monsanto’s chemical businesses are limited to sites that Solutia has owned or operated. In addition, in connection with its
spinoff that was completed in 2002, New Monsanto assumed, and agreed to indemnify Pharmacia for, any liabilities primarily related to Former
Monsanto’s chemical businesses, including, but not limited to, any such liabilities that Solutia assumed. Solutia’s and New Monsanto’s
assumption of, and agreement to, indemnify Pharmacia for these liabilities apply to pending actions and any future actions related to Former
Monsanto’s chemical businesses in which Pharmacia is named as a defendant, including, without limitation, actions asserting environmental
claims, including alleged exposure to polychlorinated biphenyls. Solutia and New Monsanto are defending and indemnifying Pharmacia in
connection with various claims and litigation arising out of, or related to, Former Monsanto’s chemical businesses.
Environmental Matters
In 2009, we submitted to the U.S. Environmental Protection Agency (EPA) a corrective measures study report with regard to Pharmacia’s
discontinued industrial chemical facility in North Haven, Connecticut and a revised site-wide feasibility study with regard to Wyeth Holdings
Corporation’s discontinued industrial chemical facility in Bound Brook, New Jersey. In September 2010, our corrective measures study report
with regard to the North Haven facility was approved by the EPA, and we commenced construction of the site remedy in late 2011 under an
Updated Administrative Order on Consent with the EPA. In July 2011, Wyeth Holdings Corporation finalized an Administrative Settlement
Agreement and Order on Consent for Removal Action with the EPA with regard to the Bound Brook facility. In May 2012, we completed
construction of an interim remedy to address the discharge of impacted groundwater from that facility to the Raritan River. In September 2012,
the EPA issued a final remediation plan for the Bound Brook facility’s main plant area, which is generally in accordance with one of the
remedies evaluated in our revised site-wide feasibility study. In March 2013, Wyeth Holdings Corporation (now Wyeth Holdings LLC) entered
into an Administrative Settlement Agreement and Order on Consent with the EPA to allow us to undertake detailed engineering design of the
remedy for the main plant area and to perform a focused feasibility study for two adjacent lagoons. In September 2015, the U.S., on behalf of
the EPA, lodged a complaint and consent decree with the federal District Court for the District of New Jersey that will allow Wyeth Holdings
LLC to complete the design and to implement the remedy for the main plant area. In December 2015, the consent decree was entered by the
District Court. The estimated costs of the site remedy for the North Haven facility and the site remediation for the Bound Brook facility are
covered by accruals previously taken by us.
India’s National Green Tribunal (NGT) and the Maharashtra Pollution Control Board (MPCB) are actively reviewing various industrial facilities
in the vicinity of Aurangabad, India, to determine whether those facilities have contributed to alleged groundwater and soil contamination in the
area. In July 2015, the NGT issued an order directing Hospira India, as the owner of a manufacturing facility in Aurangabad, to deposit
approximately $1.8 million in escrow (subsequently reduced to $0.9 million) to be applied to any required costs of remediation in the event
Hospira India is determined to have responsibility for the alleged contamination. Subsequent to the NGT order, MPCB ordered the immediate
closure of Hospira India’s Aurangabad facility. Hospira India appealed the MPCB order and in November 2015, the closure order was
overturned by the NGT.
We are a party to a number of other proceedings brought under the Comprehensive Environmental Response, Compensation, and Liability Act
of 1980, as amended, and other state, local or foreign laws in which the primary relief sought is the cost of past and/or future remediation.
A4. Legal Proceedings––Government Investigations
Like other pharmaceutical companies, we are subject to investigations and extensive regulation by government agencies in the U.S., other
developed markets and multiple emerging markets in which we operate. As a result, we have interactions with government agencies on an
ongoing basis. Criminal charges, and substantial fines and/or civil penalties, as well as limitations on our ability to conduct business in
applicable jurisdictions, could result from government investigations. Among the investigations by government agencies are the matters
discussed below.
In 2009, the U.S. Department of Justice (DOJ) filed a civil complaint in intervention in two qui tam actions that had been filed under seal in the
U.S. District Court for the District of Massachusetts. The complaint alleges that Wyeth’s practices relating to the pricing for Protonix for
Medicaid rebate purposes between 2001 and 2006, prior to Wyeth’s acquisition by Pfizer, violated the Federal Civil False Claims Act and
federal common law. The two qui tam actions have been unsealed and the complaints include substantially similar allegations. In addition, in
2009, several states and the District of Columbia filed a complaint under the same docket number in the U.S. District Court for the District of
Massachusetts asserting violations of various state laws based on allegations substantially similar to those set forth in the civil complaint filed
by the DOJ. On February 12, 2016, Wyeth and the DOJ reached an agreement in principle to resolve the actions pending in the U.S. District
Court for the District of Massachusetts for $784.6 million. The agreement in principle does not include an admission of liability by Wyeth and is
subject to the negotiation of final settlement agreements and court approval.