Pfizer 2015 Annual Report Download - page 29
Download and view the complete annual report
Please find page 29 of the 2015 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
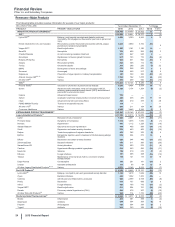
Pfizer Inc. and Subsidiary Companies
28
2015 Financial Report
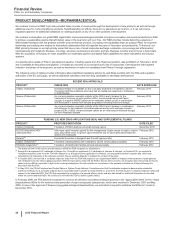
PRODUCT DEVELOPMENTS—BIOPHARMACEUTICAL
We continue to invest in R&D to provide potential future sources of revenues through the development of new products, as well as through
additional uses for in-line and alliance products. Notwithstanding our efforts, there are no assurances as to when, or if, we will receive
regulatory approval for additional indications for existing products or any of our other products in development.
We continue to strengthen our global R&D organization and pursue strategies intended to improve innovation and overall productivity in R&D
to achieve a sustainable pipeline that will deliver value in the near term and over time. Our R&D priorities include delivering a pipeline of
differentiated therapies with the greatest scientific and commercial promise, innovating new capabilities that can position Pfizer for long-term
leadership and creating new models for biomedical collaboration that will expedite the pace of innovation and productivity. To that end, our
R&D primarily focuses on six high-priority areas that have a mix of small molecules and large molecules—immunology and inflammation;
cardiovascular and metabolic diseases; oncology; vaccines; neuroscience and pain; and rare diseases. Another area of focus is biosimilars.
With the acquisition of Hospira, we have expanded our biosimilars pipeline and added R&D capabilities for sterile injectables and infusion
systems.
A comprehensive update of Pfizer’s development pipeline, including assets from the Hospira acquisition, was published on February 2, 2016
and is available at www.pfizer.com/pipeline. It includes an overview of our research and a list of compounds in development with targeted
indication and phase of development, as well as mechanism of action for candidates from Phase 2 through registration.
The following series of tables provides information about significant regulatory actions by, and filings pending with, the FDA and regulatory
authorities in the EU and Japan, as well as additional indications and new drug candidates in late-stage development.
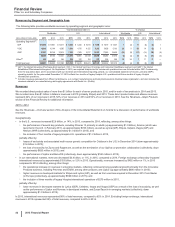
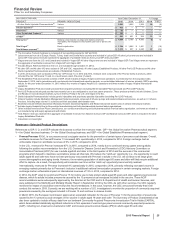
RECENT FDA APPROVALS
PRODUCT INDICATION DATE APPROVED
Xeljanz (Tofacitinib) Extended-release 11mg tablets for the once-daily treatment of moderate to severe
rheumatoid arthritis in patients who have had an inadequate response or intolerance
to methotrexate
February 2016
Ibrance (Palbociclib) An oral and selective reversible inhibitor of the CDK 4 and 6 kinases for the
treatment of hormone receptor-positive (HR+), human epidermal growth factor
receptor 2-negative (HER2-) advanced or metastatic breast cancer in combination
with fulvestrant in women with disease progression following endocrine therapy
February 2016
Ibrance (Palbociclib) An oral and selective reversible inhibitor of the CDK 4 and 6 kinases in combination
with letrozole for the treatment of postmenopausal women with estrogen receptor-
positive (ER+), HER2- advanced breast cancer as an initial endocrine-based therapy
for their metastatic disease
February 2015
PENDING U.S. NEW DRUG APPLICATIONS (NDA) AND SUPPLEMENTAL FILINGS
PRODUCT PROPOSED INDICATION DATE FILED*
Xalkori (Crizotinib) Treatment of ROS1-positive non-small cell lung cancer December 2015
ALO-02 (oxycodone HCI/
naltrexone/HCI)
A Mu-type opioid receptor agonist for the management of pain severe enough to require
daily, around-the-clock, long-term opioid treatment and for which alternative treatment
options are inadequate
February 2015
Retacrit(a) A potential biosimilar to Epogen® and Procrit® (epotein alfa) February 2015
Xeljanz (Tofacitinib)(b) Treatment of adult patients with moderate to severe chronic plaque psoriasis February 2015
Tafamidis meglumine(c) Treatment of transthyretin familial amyloid polyneuropathy February 2012
* The dates set forth in this column are the dates on which the FDA accepted our submissions.
(a) Epogen® is a registered U.S. trademark of Amgen Inc.; Procrit® is a registered U.S. trademark of Johnson & Johnson. In October 2015, we received a
“complete response” letter from the FDA with respect to our biologics license application for Retacrit, our proposed biosimilar to epoetin alfa, which was
submitted for all indications of the reference product. We are working diligently to address the content of the letter.
(b) In October 2015, we received a “complete response” letter from the FDA with respect to our supplemental NDA for Xeljanz for the treatment of adult patients
with moderate to severe chronic plaque psoriasis. While we have yet to meet with the FDA to discuss their concerns, we recognize that overcoming the issues
raised may be difficult, especially in light of the evolving marketplace. We will consider our investment in the psoriasis indication for Xeljanz following this
discussion with the FDA.
(c) In May 2012, the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee voted that the tafamidis meglumine data provide substantial
evidence of efficacy for a surrogate endpoint that is reasonably likely to predict a clinical benefit. In June 2012, the FDA issued a “complete response” letter with
respect to the tafamidis NDA. The FDA has requested the completion of a second efficacy study, and also has asked for additional information on the data
within the current tafamidis NDA. We continue to work with the FDA to define a path forward.
In February 2008, the FDA advised it expected to convene an advisory committee pending responses to the “approvable letters” for the Viviant
(bazedoxifene) NDAs for the treatment and prevention of post-menopausal osteoporosis, which were received in December 2007 and May
2008. In view of the approval of Duavee (conjugated estrogens/bazedoxifene), we submitted a request to withdraw the NDAs for Viviant in
December 2015.