Medtronic 2014 Annual Report Download - page 58
Download and view the complete annual report
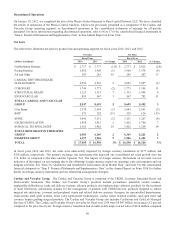
Please find page 58 of the 2014 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.Acquisition-Related Items During fiscal year 2014, we recorded net charges from acquisition-related items of $117 million,
primarily including IPR&D and long-lived asset impairment charges of $236 million related to the Ardian, Inc. (Ardian)
acquisition and income of $(138) million related to the change in fair value of contingent consideration associated with
acquisitions subsequent to April 29, 2009. The Ardian impairment resulted from our January 2014 announcement that the U.S.
pivotal trial in renal denervation for treatment-resistant hypertension, Symplicity HTN-3, failed to meet its primary efficacy
endpoint. Based on the results of the trial, we suspended enrollment of our renal denervation hypertension trials that were being
conducted in the U.S., Japan, and India. These impairment charges consisted of $192 million related to IPR&D and $44 million
related to other long-lived assets. For additional information regarding these impairment assessments, refer to Note 6 to the
consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on
Form 10-K. The change in fair value of contingent consideration primarily related to adjustments for Ardian, which are based
on annual revenue growth through fiscal year 2015. As there is no projected revenue growth through fiscal year 2015, no
contingent consideration remained as of April 25, 2014.
During fiscal year 2013, we recorded net income from acquisition-related items of $49 million, primarily including income of
$62 million related to the change in fair value of contingent consideration associated with acquisitions subsequent to April 29,
2009. The change in fair value of contingent consideration primarily related to the reduction in fair value of contingent
consideration associated with Ardian due to a slower commercial ramp in Europe. Additionally, we recorded transaction-related
expenses of $13 million.
During fiscal year 2012, we recorded net charges from acquisition-related items of $12 million, primarily including $45 million
of charges related to the change in fair value of contingent consideration associated with acquisitions subsequent to April 29,
2009. In connection with the acquisitions of Salient and PEAK, we recognized gains of $32 million and $6 million, respectively,
on our previously-held investments.
See Note 4 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual
Report on Form 10-K for further discussion on IPR&D charges.
We are responsible for the valuation of IPR&D charges. The values assigned to IPR&D are based on valuations that have been
prepared using methodologies and valuation techniques consistent with those used by independent appraisers. All values were
determined by identifying research projects in areas for which technological feasibility had not been established. Additionally,
the values were determined by estimating the revenue and expenses associated with a project’s sales cycle and the amount of
after-tax cash flows attributable to these projects. The future cash flows were discounted to present value utilizing an
appropriate risk-adjusted rate of return. The rate of return included a factor that takes into account the uncertainty surrounding
the successful development of the IPR&D.
At the time of acquisition, we expect that all acquired IPR&D will reach technological feasibility, but there can be no assurance
that the commercial viability of these products will actually be achieved. The nature of the efforts to develop the acquired
technologies into commercially viable products consists principally of planning, designing, and conducting clinical trials
necessary to obtain regulatory approvals. The risks associated with achieving commercialization include, but are not limited to,
delay or failure to obtain regulatory approvals to conduct clinical trials, delay or failure to obtain required market clearances, or
delays or issues with patent issuance, or validity and litigation. If commercial viability were not achieved, we would likely look
to other alternatives to provide these therapies.
See the “Acquisitions” section of this management’s discussion and analysis for detailed discussion of each material acquisition
in fiscal years 2014, 2013, and 2012.
Certain Tax Adjustments In fiscal year 2014, we recorded a $63 million certain tax benefit associated with the resolution of
certain issues in the fourth quarter of fiscal year 2014 with the U.S. Internal Revenue Service (IRS) relating to their review of
our fiscal year 2009 through 2011 domestic income tax returns. The $63 million certain tax benefit was recorded in the
provision for income taxes in the consolidated statement of earnings for fiscal year 2014.
In fiscal years 2013 and 2012, there were no certain tax adjustments.
See the “Income Taxes” section of this management’s discussion and analysis for further discussion of the certain tax
adjustments.
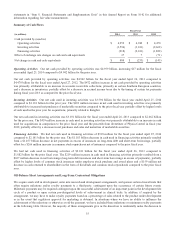
Amortization of Intangible Assets Amortization of intangible assets includes the amortization expense of our definite-lived
intangible assets consisting of purchased patents, trademarks, tradenames, purchased technology, and other intangible assets. In
50