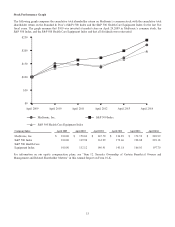
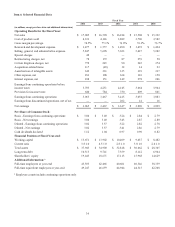
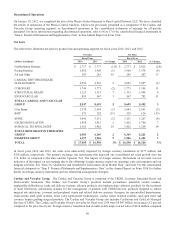
Medtronic 2014 Annual Report Download - page 51
Download and view the complete annual report
Please find page 51 of the 2014 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.•Continued acceptance and future growth from the Viva/Brava family of CRT-D devices and the Attain
Performa portfolio of quadripolar leads. The Viva/Brava family of CRT-D devices utilizes a new algorithm,
called AdaptivCRT, which improves patients’ response rates to CRT-D therapy by preserving the patients’
normal heart rhythms and continually adapts to individual patient needs. Our Viva/Brava CRT-D devices
received CE Mark approval in August 2012, received U.S. FDA approval in May 2013, and launched in Japan
in the third quarter of fiscal year 2014. Paired with Viva/Brava Quad CRT-D, Attain Performa leads provide
additional options for physicians to optimize patient therapy. Our Attain Performa left-heart leads received CE
Mark approval in March 2013 and launched in Japan in the third quarter of fiscal year 2014.
•Continued acceptance and future growth from the Advisa DR MRI SureScan pacing system. The Advisa
DR MRI SureScan is our second-generation MRI pacing system and is the first system to combine advanced
pacing technology with proven MRI access. The Advisa DR MRI SureScan was launched in Europe during
the fourth quarter of fiscal year 2010, in Japan in the second quarter of fiscal year 2013, and in the U.S. in
February 2013. In the third quarter of fiscal year 2014, we received expanded labeling for full-body MRI
scans from the U.S. FDA.
•Acceptance of Cardiocom’s integrated solutions for the management of chronic diseases such as heart failure,
diabetes, and hypertension. Cardiocom was acquired in August 2013. In the third quarter of fiscal year 2014,
Cardiocom launched Re30, a 30-day readmission reduction program focused on minimizing heart failure
readmission penalties for U.S. hospitals.
•Acceptance of our CLMS business. CLMS provides a unique service offering, whereby we enter into long-
term contracts with hospitals to upgrade and more effectively manage their cath lab and hybrid operating
rooms.
•Continued evaluation of the long-term strategy of our renal denervation therapy. In January 2014, we
announced our U.S. pivotal trial in renal denervation for treatment-resistant hypertension, Symplicity HTN-3,
failed to meet its primary efficacy endpoint, while its primary safety endpoint was achieved. Based on the
results of the trial, we have suspended enrollment of our renal denervation hypertension trials that were being
conducted in the U.S., Japan, and India. We will continue to provide access to the Symplicity system in
countries where it has regulatory approval and we remain in discussions with the U.S. FDA regarding a
potential approval path for the U.S.
•Continued acceptance of the Resolute Integrity drug-eluting coronary stent and the Integrity bare metal stent.
In February 2013, the U.S. FDA approved longer lengths of our Resolute Integrity drug-eluting coronary
stent, providing access to a larger portion of the U.S. drug-eluting stent market. We launched small vessel
sizes and longer lengths of our Resolute Integrity drug-eluting coronary stent in Japan during the second and
third quarters of fiscal year 2014, respectively. The global stent market continues to experience year-over-year
declines, including increasing pricing pressure resulting from government austerity programs and
reimbursement cuts in Western Europe, Japan, and India.
•Continued acceptance of our CoreValve transcatheter heart valve technologies for the replacement of the
aortic valve. The CoreValve 31 millimeter received CE Mark approval in the first quarter of fiscal year 2012.
The CoreValve Evolut 23 millimeter valve, which promotes better sealing and provides future recapturability,
was launched in Europe in the first quarter of fiscal year 2013. The CoreValve System received CE Mark
approval and is currently available outside the U.S. Late in the third quarter of fiscal year 2014, we received
U.S. FDA approval for our CoreValve transcatheter aortic heart valve for extreme risk patients in the U.S. We
received U.S. approval for high risk patients in June 2014. Additionally, CoreValve related patent litigation
with Edwards was settled in May 2014, requiring ongoing royalty payments through April 2022. For
additional information, see Note 18 to the consolidated financial statements in “Item 8. Financial Statements
and Supplementary Data” in this Annual Report on Form 10-K.
•Continued worldwide growth of the Valiant Captivia Thoracic Stent Graft System. The Valiant Captivia
Thoracic Stent Graft System was launched in the U.S. in the fourth quarter of fiscal year 2012 and in Japan
and China in the first quarter of fiscal year 2013. We received U.S. FDA approval of a dissection indication
for the Valiant Captivia Thoracic Stent Graft System in January 2014.
43