Medtronic 2014 Annual Report Download - page 48
Download and view the complete annual report
Please find page 48 of the 2014 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Discontinued Operations
On January 30, 2012, we completed the sale of the Physio-Control business to Bain Capital Partners, LLC. We have classified
the results of operations of the Physio-Control business, which were previously presented as a component of the Cardiac and
Vascular Group operating segment, as discontinued operations in the consolidated statements of earnings for all periods
presented. For more information regarding discontinued operations, refer to Note 17 to the consolidated financial statements in
“Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K.
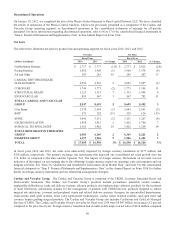
Net Sales
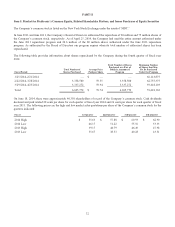
The table below illustrates net sales by product line and operating segment for fiscal years 2014, 2013, and 2012:
Net Sales Net Sales
Fiscal Year Fiscal Year
(dollars in millions) 2014 2013 % Change 2013 2012 % Change
Defibrillation Systems $ 2,757 $ 2,773 (1)% $ 2,773 $ 2,822 (2)%
Pacing Systems 1,892 1,906 (1) 1,906 1,978 (4)
AF and Other 347 243 43 243 207 17
CARDIAC RHYTHM DISEASE
MANAGEMENT 4,996 4,922 2 4,922 5,007 (2)
CORONARY 1,744 1,773 (2) 1,773 1,598 11
STRUCTURAL HEART 1,212 1,133 7 1,133 1,094 4
ENDOVASCULAR 895 867 3 867 783 11
TOTAL CARDIAC AND VASCULAR
GROUP 8,847 8,695 2 8,695 8,482 3
Core Spine 2,570 2,603 (1) 2,603 2,643 (2)
BMP 471 528 (11) 528 624 (15)
SPINE 3,041 3,131 (3) 3,131 3,267 (4)
NEUROMODULATION 1,898 1,812 5 1,812 1,700 7
SURGICAL TECHNOLOGIES 1,562 1,426 10 1,426 1,254 14
TOTAL RESTORATIVE THERAPIES
GROUP 6,501 6,369 2 6,369 6,221 2
DIABETES GROUP 1,657 1,526 9 1,526 1,481 3
TOTAL $ 17,005 $ 16,590 3% $ 16,590 $ 16,184 3%
In fiscal years 2014 and 2013, net sales were unfavorably impacted by foreign currency translation of $175 million and
$328 million, respectively. The primary exchange rate movements that impacted our consolidated net sales growth were the
U.S. dollar as compared to the Euro and the Japanese Yen. The impact of foreign currency fluctuations on net sales was not
indicative of the impact on net earnings due to the offsetting foreign currency impact on operating costs and expenses and our
hedging activities. See “Item 7A. Qualitative and Quantitative Disclosures about Market Risk” and Note 9 to the consolidated
financial statements in “Item 8. Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for further
details on foreign currency instruments and our related risk management strategies.
Cardiac and Vascular Group The Cardiac and Vascular Group is composed of the CRDM, Coronary, Structural Heart, and
Endovascular businesses. The Cardiac and Vascular Group’s products include pacemakers, insertable cardiac monitor,
implantable defibrillators, leads and delivery systems, ablation products, electrophysiology catheters, products for the treatment
of atrial fibrillation, information systems for the management of patients with CRDM devices, products designed to reduce
surgical site infections, coronary and peripheral stents and related delivery systems, therapies for uncontrolled hypertension,
endovascular stent graft systems, heart valve replacement technologies, cardiac tissue ablation systems, and open heart and
coronary bypass grafting surgical products. The Cardiac and Vascular Group also includes Cardiocom and Cath Lab Managed
Services (CLMS). The Cardiac and Vascular Group’s net sales for fiscal year 2014 were $8.847 billion, an increase of 2 percent
compared to the prior fiscal year. Foreign currency translation had an unfavorable impact on net sales of $118 million compared
40