Medtronic 2014 Annual Report Download - page 47
Download and view the complete annual report
Please find page 47 of the 2014 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.acquired as part of a business combination requires us to make significant estimates. These estimates include the amount and
timing of projected future cash flows, the discount rate used to discount those cash flows to present value, the assessment of the
asset’s life cycle, and the consideration of legal, technical, regulatory, economic, and competitive risks. The fair value assigned
to other intangible assets, including IPR&D, is determined by estimating the future cash flows of each project or technology and
discounting the net cash flows back to their present values. The discount rate used is determined at the time of measurement in
accordance with accepted valuation methodologies.
IPR&D acquired in a business combination is capitalized as an indefinite-lived intangible asset. Development costs incurred
after the acquisition are expensed as incurred. Upon receipt of regulatory approval, the indefinite-lived intangible asset is then
accounted for as a finite-lived intangible asset and amortized on a straight-line basis or accelerated basis, as appropriate, over its
estimated useful life. If the R&D project is subsequently abandoned, the indefinite-lived intangible asset is charged to expense.
IPR&D acquired outside of a business combination is expensed immediately.
Due to the uncertainty associated with R&D projects, there is risk that actual results will differ materially from the original cash
flow projections and that the R&D project will result in a successful commercial product. The risks associated with achieving
commercialization include, but are not limited to, delay or failure to obtain regulatory approvals to conduct clinical trials, delay
or failure to obtain required market clearances, delays or issues with patent issuance, or validity and litigation.
Goodwill is the excess of the purchase price (consideration) over the estimated fair value of net assets, including IPR&D, of
acquired businesses. Goodwill is tested for impairment annually in the third quarter and whenever an event occurs or
circumstances change that would indicate that the carrying amount may be impaired. The test for impairment requires us to
make several estimates about fair value, most of which are based on projected future cash flows. Our estimates associated with
the goodwill impairment test are considered critical due to the amount of goodwill recorded on our consolidated balance sheets
and the judgment required in determining fair value, including projected future cash flows. The results of our annual impairment
test are discussed in Note 6 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data”
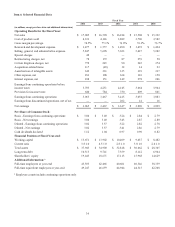
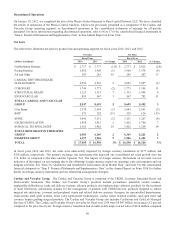
in this Annual Report on Form 10-K. Goodwill was $10.593 billion and $10.329 billion as of April 25, 2014 and April 26, 2013,
respectively.
Other intangible assets include patents, trademarks, purchased technology, and IPR&D (since April 25, 2009). Intangible assets
with a definite life are amortized on a straight-line or accelerated basis, as appropriate, with estimated useful lives ranging from
three to 20 years. IPR&D is tested for impairment annually in the third quarter and whenever an event occurs or circumstances
change that would indicate that the carrying amount may be impaired. We review other definite-lived intangible assets for
impairment whenever events or circumstances indicate that the carrying amount of an asset (asset group) may not be
recoverable. Refer to Note 1 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data”
in this Annual Report on Form 10-K for additional information. Our impairment reviews are based on an estimated future cash
flow approach that requires significant judgment with respect to future revenue and expense growth rates, selection of
appropriate discount rate, asset groupings, and other assumptions and estimates. We use estimates that are consistent with our
business plans and a market participant view of the assets being evaluated. The results of our annual impairment test are
discussed in Note 6 to the consolidated financial statements in “Item 8. Financial Statements and Supplementary Data” in this
Annual Report on Form 10-K. Actual results may differ from our estimates due to a number of factors including, among others,
changes in competitive conditions, timing of regulatory approval, results of clinical trials, changes in worldwide economic
conditions, and fluctuations in foreign currency exchange rates. These risk factors are discussed in “Item 1A. Risk Factors” in
this Annual Report on Form 10-K. Other intangible assets, net of accumulated amortization, were $2.286 billion and
$2.673 billion as of April 25, 2014 and April 26, 2013, respectively.
Contingent consideration is recorded at the acquisition date at the estimated fair value of the contingent consideration for all
acquisitions subsequent to April 24, 2009. The acquisition date fair value is measured based on the consideration expected to be
transferred (probability-weighted), discounted back to present value. The discount rate used is determined at the time of
measurement in accordance with accepted valuation methodologies. The fair value of the contingent consideration is
remeasured at the estimated fair value at each reporting period with the change in fair value recognized as income or expense
within acquisition-related items in our consolidated statements of earnings. Changes to the fair value of contingent
consideration can result from changes in discount rates, the timing and amount of revenue estimates, or in the timing or
likelihood of achieving the milestones which trigger payment. Using different valuation assumptions including revenue or cash
flow projections, growth rates, discount rates, or probabilities of achieving the milestones result in different fair value
measurements, future amortization expense, and expense in the current or future periods. The fair value of contingent
consideration was $68 million and $142 million as of April 25, 2014 and April 26, 2013, respectively.
39