Medtronic 2014 Annual Report Download - page 130
Download and view the complete annual report
Please find page 130 of the 2014 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Medtronic, Inc.
Notes to Consolidated Financial Statements (Continued)
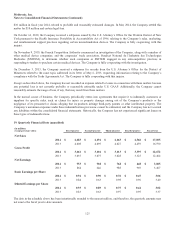
The following is a summary of the operating results of Physio-Control for discontinued operations for fiscal year 2012:
(in millions) 2012
Discontinued operations:
Net sales $ 323
Earnings from operations of Physio-Control $ 48
Physio-Control divestiture-related costs (42)
Gain on sale of Physio-Control 218
Income tax expense (22)
Earnings from discontinued operations $ 202
In the fourth quarter of fiscal year 2012, the Company recognized a pre-tax gain on sale of $218 million, which included a
reversal of the portion of the Company’s currency translation adjustment related to Physio-Control. Additionally, during fiscal
year 2012, the Company recorded $42 million of Physio-Control divestiture-related costs in discontinued operations. The
Company reclassified $12 million of Physio-Control divestiture-related costs previously recorded in acquisition-related items
within continuing operations on the consolidated statements of earnings in the first and second quarters of fiscal year 2012 to
discontinued operations.
18. Contingencies
The Company is involved in a number of legal actions. The outcomes of these legal actions are not within the Company’s
complete control and may not be known for prolonged periods of time. In some actions, the claimants seek damages, as well as
other relief (including injunctions barring the sale of products that are the subject of the lawsuit), that could require significant
expenditures or result in lost revenues. In accordance with U.S. GAAP, the Company records a liability in the consolidated
financial statements for loss contingencies when a loss is known or considered probable and the amount can be reasonably
estimated. If the reasonable estimate of a known or probable loss is a range, and no amount within the range is a better estimate
than any other, the minimum amount of the range is accrued. If a loss is reasonably possible but not known or probable, and can
be reasonably estimated, the estimated loss or range of loss is disclosed. When determining the estimated loss or range of loss,
significant judgment is required to estimate the amount and timing of a loss to be recorded. Estimates of probable losses
resulting from litigation and governmental proceedings involving the Company are inherently difficult to predict, particularly
when the matters are in early procedural stages, with incomplete scientific facts or legal discovery; involve unsubstantiated or
indeterminate claims for damages; potentially involve penalties, fines or punitive damages; or could result in a change in
business practice. While it is not possible to predict the outcome for most of the matters discussed, the Company believes it is
possible that costs associated with them could have a material adverse impact on the Company’s consolidated earnings,
financial position, or cash flows.
Litigation with Wyeth and Cordis Corporation
On February 22, 2008, Wyeth and Cordis Corporation (Cordis) filed a lawsuit against the Company and its subsidiary,
Medtronic AVE, Inc., in U.S. District Court for the District of New Jersey, alleging that Medtronic’s Endeavor drug-eluting
stent infringes three U.S. “Morris” patents alleged to be owned by Wyeth and exclusively licensed to Cordis. On January 19,
2012, the Court found the patent claims asserted against Medtronic to be invalid and entered an Order and Judgment in favor of
Medtronic and the other defendants. Wyeth and Cordis have appealed. On June 24, 2013, the Court of Appeals for the Federal
Circuit affirmed the District Court’s order. The Company is indemnified for the claims made by Wyeth and Cordis. The
Company has not recorded an expense related to damages in connection with these matters because any potential loss is not
currently probable or reasonably estimable under U.S. GAAP. Additionally, the Company cannot reasonably estimate the range
of loss, if any, that may result from this matter.
Litigation with Edwards Lifesciences Corporation
On March 19, 2010, the U.S. District Court for the District of Delaware added Medtronic CoreValve LLC (CoreValve) as a
party to litigation pending between Edwards and CoreValve, Inc. In the litigation, Edwards asserted that CoreValve’s
122