Medtronic 2014 Annual Report Download - page 57
Download and view the complete annual report
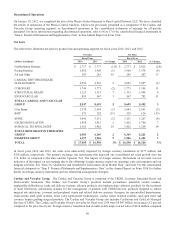
Please find page 57 of the 2014 Medtronic annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.Fiscal Year 2013 Initiative
The fiscal year 2013 initiative was designed to scale back our infrastructure in slower growing areas of our business, while
continuing to invest in geographies, businesses, and products where we anticipate faster growth. A number of factors have
contributed to ongoing challenging market dynamics, including increased pricing pressure, various governmental austerity
measures, and the U.S. medical device excise tax. In the fourth quarter of fiscal year 2013, we recorded a $192 million
restructuring charge, which consisted of employee termination costs of $150 million, asset write-downs of $13 million, contract
termination costs of $18 million, and other related costs of $11 million. Of the $13 million of asset write-downs, $10 million
related to inventory write-offs of discontinued product lines and production-related asset impairments, and therefore, was
recorded within cost of products sold in the consolidated statements of earnings. In the first quarter of fiscal year 2014, we
recorded an $18 million restructuring charge, which was the final charge related to the fiscal year 2013 initiative and consisted
primarily of contract termination costs of $14 million and other related costs of $4 million.
As of the end of the fourth quarter of fiscal year 2013, we identified approximately 2,000 positions for elimination to be
achieved through involuntary and voluntary separation.
In fiscal year 2014, we recorded a $46 million reversal of excess restructuring reserves related to the fiscal year 2013 initiative.
The reversal was primarily a result of revisions to particular strategies and certain employees identified for elimination finding
other positions within the Company.
As a result of certain legal requirements outside the U.S., the fiscal year 2013 initiative is scheduled to be substantially complete
by the end of the third quarter of fiscal year 2016.
Fiscal Year 2012 Initiative
In the fourth quarter of fiscal year 2012, we recorded a $118 million restructuring charge, which consisted of employee
termination costs of $66 million, asset write-downs of $9 million, contract termination costs of $30 million, and other related
costs of $13 million. The fiscal year 2012 initiative was designed to reduce general, administrative, and indirect distribution
costs in certain organizations within the Company while prioritizing investment in research and development, and sales and
marketing in those organizations within the Company where faster growth is anticipated, such as emerging markets and new
technologies.
As of the end of the fourth quarter of fiscal year 2012, we identified approximately 1,000 positions for elimination to be
achieved through involuntary and voluntary separation. As of April 26, 2013, the fiscal year 2012 initiative was substantially
complete.
In the fourth quarter of fiscal year 2013, we recorded a $10 million reversal of excess restructuring reserves related to the fiscal
year 2012 initiative. This reversal was primarily a result of revisions to particular strategies and certain employees identified for
elimination finding other positions within the Company.
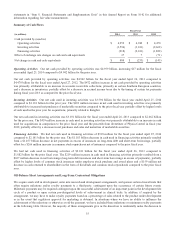
For additional information, see Note 3 to the consolidated financial statements in “Item 8. Financial Statements and
Supplementary Data” in this Annual Report on Form 10-K.
Certain Litigation Charges, Net We classify material litigation charges and gains recognized as certain litigation charges, net.
During fiscal year 2014, we recorded certain litigation charges, net of $770 million, which primarily includes the global patent
settlement agreement with Edwards of $589 million, accounting charges for probable and reasonably estimable INFUSE
product liability litigation of $140 million, and other litigation. See Note 18 to the consolidated financial statements in “Item 8.
Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for additional information.
During fiscal year 2013, we recorded certain litigation charges, net of $245 million related to probable and reasonably estimated
damages resulting from patent litigation with Edwards. See Note 18 to the consolidated financial statements in “Item 8.
Financial Statements and Supplementary Data” in this Annual Report on Form 10-K for additional information.
During fiscal year 2012, we recorded certain litigation charges, net of $90 million related to the agreement to settle the federal
securities class action initiated in December 2008 by the Minneapolis Firefighters’ Relief Association. During the fourth quarter of
fiscal year 2012, Medtronic settled all of these class claims for $85 million and incurred $5 million in additional litigation fees.
49