Pentax 2009 Annual Report Download - page 35
Download and view the complete annual report
Please find page 35 of the 2009 Pentax annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
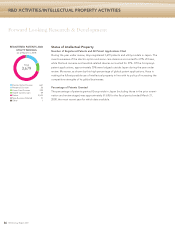
Artificial Crystalline Lens Material: Recognition from the Japan Opthalmological Society
In the field of ophthalmology, Hoya is conducting R&D of artificial materials for use inside
crystalline lens capsules. Animal trials are already underway, and are yielding extremely posi-
tive results. This material offers the potential for vision correction, and in the future may have
medical applications that rival that of intraocular lenses.
Scanning Fiber Endoscope (SFE): Completion of Ultra-Small Diameter Endoscope Prototype
SFE is a new type of imaging device developed jointly by the University of Washington and
Pentax. An ultra-small diameter prototype endoscope created using this technology is cur-
rently under testing. SFE produces high-definition images that are equal to or better than
those provided by charge-coupled devices used in current endoscopes, while achieving a
high frame rate.*2 Using a narrow-band laser light source and image processing, the Company
is working to improve the ability to distinguish between normal areas and tumors, and in com-
bination with various optical technologies, it is aiming for application in applied products with
new functionality.
*2 Frame rate is a standard measure of frequency per time unit that an image is refreshed. Higher frame rates generate
smoother images.
Ultrasonic Bronchoscope: Commercialization in October 2008
Much like an electronic endoscope, bronchoscopes are equipped with a color CCD on one
end, allowing the medical professional to view the image on the screen as the device is
inserted into the bronchial area. The convex array transducer enables ultrasound imaging
from the tracheal mucous membrane. In October 2008, Hoya launched sales of the world’s
first ultrasonic bronchoscope, EB-1970UK, for the European market. This product enables
physicians to view a precise optical image of the target area and then use the ultrasound
image to confirm the affected area while making punctures. This new technology is expected
to be effective in the diagnosis and treatment of chest diseases such as lung cancer.
Biocompatible Organic/Inorganic Composite Bone Prostheses: Proven Effective in Clinical Trials
When implanted in the human body, biocompatible organic/inorganic composite bone pros-
theses are absorbed through the same mechanism as normal bone metabolism to form new
bone tissue because they have the same structure as natural human bone. These bone pros-
theses are garnering attention as a bone regeneration “scaffolding material” that is
approaching practical application in regenerative medicine. The prostheses show promise in
applications that unite them with stem cells and bone morphogenetic protein technologies.
Head of an ultra-small diameter
prototype SFE
Artificial Crystalline Lens Material
The EB-1970UK ultrasonic
bronchoscope
Injection (Gel)
Gel
Crystalline lens capsule
The resilience of biocompatible
organic/inorganic composite bone
prostheses
33
HOYA Annual Report 2009