Pentax 2009 Annual Report Download - page 22
Download and view the complete annual report
Please find page 22 of the 2009 Pentax annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
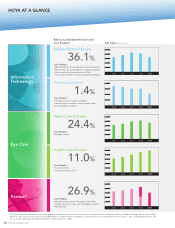
INTRAOCULAR LENSES (IOL)
Fiscal 2009 Business Overview and Results
Intraocular lenses (IOL) are artificial lenses used to replace clouded
lenses during cataract surgery. Inserting these lenses into the
affected eye can restore vision. Over many years of research into
optical design and high- function materials, Hoya develops and
provides unique IOL products that meet the needs of doctors prac-
ticing on the frontlines of medicine.
Hoya’s foldable soft lenses can be folded into a small package,
enabling delivery into the eye through a very small 1.8mm incision,
the smallest in the world. Moreover, the pre-loaded iSert delivery
system, which offers superior operability compared to previous
ones, is contributing to the progress of medicine as a solution for
enabling safe, accurate cataract procedures.
During the fiscal year under review, Hoya launched an aspheri-
cal pre-loaded IOL in the Japanese market. With focus on introduc-
tion and sales of the new product to medical institutions of all
types, the Company was able to maintain strong sales from the
previous fiscal year.
Outside of Japan, the Company focused on the launch of ultra-
small incision type IOLs and expanding sales of the iSert system in
developed countries, mainly Germany and France, and succeeded
in raising market presence. In the Asia-Pacific region, there was
steady progress in introducing new products, with a focus on China,
South Korea and Australia. These measures resulted in high growth
and strong results.
In addition, as business expanded, the Company conducted
construction to expand production capacity in the Singapore fac-
tory, strove to secure a low-cost, stable supply framework, and
pressed ahead with development and trial production of new prod-
ucts and materials for IOLs.
In September 2008, the iSpheric™ yellow acrylic foldable intraoc-
ular lens was approved by the U.S. Food and Drug Administration
(FDA), and Hoya became the first Japanese manufacturer to enter
the U.S. market for intraocular lenses, estimated to be the largest in
the world. In April 2009, the Company began more full-fledged mar-
keting activities in the United States. In the previous fiscal year, Hoya
transferred its operations headquarters to Southern California, where
world-renowned ophthalmologists conduct cutting-edge research.
Taking FDA approval of the lenses as the impetus to accelerate cre-
ation of a sales structure in the United States, Hoya is now working to
expand its business with a global focus.
Future Strategies
As society ages, the number of cataract suffers in developed coun-
tries is rising sharply. Developing countries are also seeing growth
in the number of cataract patients seeking care as their economies
rapidly expand. IOLs are therefore expected to see market growth
worldwide. Hoya will pursue this growth opportunity by promoting
R&D to enhance product functionality.
Yellow acrylic intraocular lens
These yellow intraocular lenses
absorb ultra-violet light, are
easy on the retina, and provide
natural color tones. In 1991,
Hoya became the first in the
industry to release colored
intraocular lenses.
The pre-loaded iSert delivery
system
This disposable injector comes
with an intraocular lens pre-
loaded. In addition to reducing
the amount of work required
during surgery, it does not
require washing or sterilization
of related tools, facilitating
cataract surgery.
EYE CARE
20 HOYA Annual Report 2009