Pentax 2008 Annual Report Download - page 6
Download and view the complete annual report
Please find page 6 of the 2008 Pentax annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
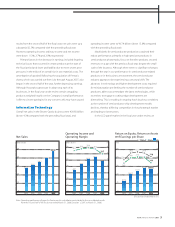
emphasis by panel manufacturers on mass production instead of
new development softened orders for new masks from photomask
producers including Hoya. Consequently, the photomask market
contracted even as the panel market expanded. Although the
Company seems to have slightly augmented its market share, the
shrinkage of the market as a whole made it difficult to maintain
sales levels. At the same time, we are achieving satisfactory results
from our efforts in recent years to technologically differentiate our
products. Such efforts are progressing steadily and have resulted
in higher ratings from customers.
In glass disks for hard disk drives, the Company got an early
start on technical development relating to the perpendicular
magnetic recording method, on which HDD producers
commenced full-scale commercialization from the end of the
preceding fiscal year. When we began manufacturing the new
product, it unfortunately took longer than anticipated to develop
technologies in line with the demands of our clients, leading to
disappointing results in the first half of the fiscal year. However,
development gradually caught up and performance staged a
healthy recovery in the third and fourth quarters. We are still not
making the most of our potential, and we believe we must make
swift changes to our operations to take full advantage of our
capacity in technology and production.
Concerning optical lenses, the digital camera market—which
has grown for several years almost entirely without seasonal
adjustments—was steady through the third quarter of the fiscal
year. Although the Company’s performance was weakened in the
fourth quarter by the influence of short-term production
adjustments carried out by digital camera manufacturers, results
for the full fiscal year remained firm. The digital camera market is
expected to continue expanding, with the number of units shipped
rising in line with growing demand from emerging markets. We
expect unit prices to fall, however, causing the growth rate to taper
off. Hoya will continue to pursue its policy of market growth through
specialization in products that utilize its technical capabilities.
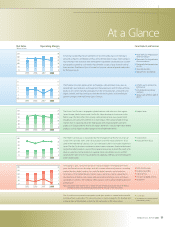
Eye Care
In the Vision Care business, which produces eyeglass lenses, net
sales totaled ¥126.3 billion (up 5.5% compared with the preceding
fiscal year) and operating income reached ¥20.6 billion (down
2.4% compared with the preceding fiscal year).
The fiscal year under review witnessed improved performance
in the European market, owing to a successful expansion of sales
channels as a result of management resources dedicated to the
region, in addition to currency transaction gains from the rising
euro. In the rapidly growing Asian markets as well, we achieved
growth in the double digits and above. However, demand was
sluggish in Japan and the United States, due to the impact of
sagging consumption. Particularly in Japan, falling prices per unit
product shrank the eyeglass market itself, which blunted the
business’s overall growth rate.
Concerning the Health Care business, net sales were ¥46.1
billion (up 13.0% compared with the preceding fiscal year) and
operating income reached ¥10.1 billion (up 10.3% compared with
the preceding fiscal year).
In intraocular lenses for implantation after cataract surgery,
customers in Japan have recognized the advantages of our
products, and they are near our original goals for market share. We
expect new markets for such products once they obtain approval
from the U.S. Food and Drug Administration (FDA), which is
scheduled to happen in the year ahead. Our Eye City chain of
contact lens specialty stores in Japan slowed the pace of new
A Message to Our Stakeholders
4