Quest Diagnostics 2005 Annual Report Download
Download and view the complete annual report
Please find the complete 2005 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
»
ourfirstresponsibility
2005AnnualReport
Table of contents
-
Page 1
our฀ï¬rst฀responsibility » 2005฀Annual฀Report -
Page 2
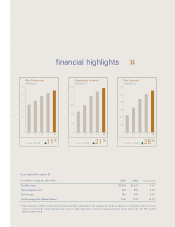
...฀in฀goodwill฀accounting.฀A฀reconciliation฀of฀these฀measures฀ and฀ the฀ most฀ directly฀ comparable฀ financial฀ measures฀ under฀ generally฀ accepted฀ accounting฀ principles฀ can฀ be฀ found฀ after฀ the฀ 2005฀ Annual฀ Report฀on฀Form฀10-K. -
Page 3
...฀centers laboratories฀focused฀on฀accurate฀and฀timely฀results clear฀strategy฀focused฀on฀Patients,฀Growth฀and฀People ฀ ฀ ฀ This฀singular฀focus฀has฀fueled฀our฀company's฀growth,฀making฀us฀the฀unrivaled฀leader฀in฀the฀diagnostic฀testing... -
Page 4
... In฀ 2005,฀ scientists฀ at฀ our฀ Quest฀ Diagnostics฀ Nichols฀ Institute฀ developed฀ Leumeta™฀ cancer฀ tests฀ to฀ provide฀ patients฀ and฀ their฀ doctors฀ with฀ vital฀ information฀ about฀ their฀disease฀using฀a฀simple-to-obtain฀blood฀sample... -
Page 5
...plan฀to฀expand฀this฀training฀to฀other฀customer฀contact฀personnel,฀such฀as฀couriers฀and฀customer฀service฀representatives We฀created฀ Quest฀Diagnostics฀Health฀Trends฀as฀a฀way฀to฀mine฀our฀enormous฀database฀of฀test฀results฀ to฀ provide... -
Page 6
... ฀ ฀ ฀ We฀supported฀these฀new฀service฀and฀science฀initiatives฀by฀providing฀our฀sales฀force฀with฀enhanced฀training฀ so฀they฀can฀become฀trusted฀advisers฀to฀our฀physician฀clients.฀In฀addition,฀we฀created฀specialized฀sales฀teams฀to... -
Page 7
...,฀creating฀proprietary฀tests,฀and฀developing฀state-of-the-art฀lab฀reports Healthcare฀information฀technology฀is฀an฀increasingly฀vital฀issue฀for฀policymakers,฀doctors,฀hospitals,฀payers฀ and฀ especially฀ patients.฀ Our฀ Care360฀ physician฀ portal... -
Page 8
» patients with฀caring 6฀»฀7 -
Page 9
...ability฀ to฀ prearrange฀ test฀ appointment฀ times฀ at฀ our฀ patient฀ service฀ centers฀ provides฀ patients฀ with฀ unsurpassed฀ convenience,฀while฀our฀kiosks฀give฀them฀easy฀access฀to฀important฀information฀about฀national฀health฀concerns฀ like... -
Page 10
...฀unique฀information฀ technology฀ platforms฀ through฀ our฀ MedPlus฀ subsidiary.฀ We฀ deliver฀ this฀ value฀ through฀ Care360,฀ an฀ Internetbased฀physician฀portal,฀which฀lets฀doctors฀order฀tests,฀view฀results฀and฀prescribe฀drugs฀electronically... -
Page 11
comes฀opportunity "I฀will฀prevent฀disease฀whenever฀I฀can,฀for฀prevention฀is฀preferable฀to฀a฀cure."฀ from฀the฀Hippocratic฀Oath » Quest฀Diagnostics -
Page 12
» people with฀responsibility 10฀»฀11 -
Page 13
...฀of฀the฀general฀public.฀Quest฀Diagnostics฀is฀aggressive฀in฀this฀regard,฀offering฀free฀testing฀at฀clinics฀such฀as฀ the฀AmeriCares฀Free฀Clinics฀in฀Connecticut฀and฀Venice฀Family฀Clinic฀in฀Los฀Angeles,฀and฀providing฀our฀services฀ to... -
Page 14
...,฀New฀Jersey Gail฀R.฀Wilensky,฀Ph.D. John฀Olin฀Senior฀Fellow Project฀HOPE Bethesda,฀Maryland Jack฀B.฀Ziegler Retired฀President฀฀ Worldwide฀Consumer฀Healthcare฀฀ GlaxoSmithKline฀plc฀ Philadelphia,฀Pennsylvania » » ฀ leadership Executive฀Officers... -
Page 15
2005 Form 10-K -
Page 16
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, DC 20549 FORM 10-K Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the Fiscal Year Ended December 31, 2005 Commission File Number 001-12215 Quest Diagnostics Incorporated 1290 Wall Street West, ... -
Page 17
... Testing ...New Test Introductions ...Risk Assessment Services ...Clinical Trials Testing ...Other Services and Products ...Payers and Customers ...Sales and Marketing ...Information Systems ...Billing ...Competition ...Quality Assurance ...Regulation of Clinical Laboratory Operations ...Healthcare... -
Page 18
...full esoteric testing services, including gene-based testing, on both coasts through our Quest Diagnostics Nichols Institute laboratory facilities, located in San Juan Capistrano, California and Chantilly, Virginia. We also have laboratory facilities in Mexico City, Mexico, San Juan, Puerto Rico and... -
Page 19
... Providing the Highest Quality Services: We strive to provide the highest quality in all that we do including: phlebotomy and specimen transport services; analytical testing processes in our laboratories; providing accurate and timely lab reports; and billing information. We use Six Sigma processes... -
Page 20
... better access to patient-centric information. We believe that these products enhance the value we provide to our customers and result in increased customer loyalty. Our Care360TM products, including our Care360 Physician Portal, enable doctors to order diagnostic tests and review laboratory results... -
Page 21
... screening and risk assessment services to life insurance companies, as well as clinical diagnostic testing services to healthcare providers and drugs-of-abuse testing to employers. LabOne operates major laboratories in Lenexa, Kansas, and Cincinnati, Ohio, as well as a state-of-the-art call center... -
Page 22
... laboratory in San Juan Capistrano, California, was the ï¬rst clinical laboratory in North America to achieve International Organization for Standardization, or ISO, 9001 certiï¬cation. Our esoteric testing laboratory in Chantilly, Virginia enables us to provide full esoteric testing services... -
Page 23
...in the United States. Our risk assessment services comprise underwriting support services to the life insurance industry including teleunderwriting, specimen collection and paramedical examinations, laboratory testing, medical record retrieval, motor vehicle reports, telephone inspections and credit... -
Page 24
... testing business during 2005 applicable to each payer group: Net Revenues as % of Total Clinical Laboratory Testing Net Revenues Requisition Volume as % of Total Volume Patient ...Medicare and Medicaid ...Physicians, Hospitals, Employers and Other Monthly-Billed Clients ...Healthcare Insurers... -
Page 25
... test utilization data in order to meet the reporting requirements of the National Committee for Quality Assurance, or NCQA, to implement disease management programs and for other health plan operation purposes. In certain markets, such as California, healthcare insurers may delegate their covered... -
Page 26
... their patients' clinical laboratory tests. We provide services to hospitals throughout the United States that vary from esoteric testing to helping manage their laboratories. We believe that we are the industry's market leader in servicing hospitals. Our hospital customers account for approximately... -
Page 27
... or inaccurate billing information provided by ordering physicians; and • disputes with payers as to which party is responsible for payment. We incur additional costs as a result of our participation in Medicare and Medicaid programs, as billing and reimbursement for clinical laboratory testing is... -
Page 28
... clinical laboratories, specialized esoteric labs, as well as laboratories owned by physicians and hospitals (see "Payers and Customers''). We believe that healthcare providers consider a number of factors when selecting a laboratory, including: • service capability and quality; • accuracy... -
Page 29
... centers. We continue to implement our Six Sigma and standardization initiatives to help achieve our goal of becoming recognized as the undisputed quality leader in the healthcare services industry. Our Nichols Institute facility in San Juan Capistrano was the ï¬rst clinical laboratory in North... -
Page 30
...states these restrictions affect our ability to directly provide anatomic pathology services and/or to provide clinical laboratory services directly to consumers. Healthcare Information Technology Clinical laboratories use information technology to obtain laboratory orders and to communicate results... -
Page 31
... regulations restrict our ability to use or disclose patient-identiï¬able laboratory data, without patient authorization, for purposes other than payment, treatment or healthcare operations (as deï¬ned by HIPAA) except for disclosures for various public policy purposes and other permitted purposes... -
Page 32
...screening tests and diabetes screening tests, subject to certain frequency limitations. The MMA evaluates new diagnostic tests for coverage as they are introduced. In addition, the 2005 Physician Fee Schedule rule proposed to lower Medicare's payment rates for ï¬,ow cytometry services in 2005. Quest... -
Page 33
..., state Medicaid programs are prohibited from paying more (and in most instances, pay signiï¬cantly less) than Medicare. Major clinical laboratories, including Quest Diagnostics, typically use two fee schedules for tests billed on a fee-for-service basis: • "Client'' fees charged to physicians... -
Page 34
... generally permitted to bill patients for clinical laboratory tests that Medicare does not cover due to "medical necessity'' limitations (these tests include limited coverage tests for which the ordering physician did not provide an appropriate diagnosis code and certain tests ordered on a patient... -
Page 35
...schedule applicable to the carrier region where the test was performed. This streamlined process allows a laboratory to ï¬le all of its clinical laboratory claims to its "home'' carrier. CMS also has announced a parallel change with regard to purchased diagnostic interpretations (pathology services... -
Page 36
..., and the government has the remedy of excluding a non-compliant provider from participation in the Medicare and Medicaid programs, which represented approximately 18% of our net revenues during 2005. We understand that there may be pending qui tam claims brought by former employees or other... -
Page 37
... of New York. Quest Diagnostics and NID have been cooperating with the United States Attorney's Ofï¬ce. In connection with such cooperation, we have been providing information and producing various business records of NID and Quest Diagnostics, including documents related to testing and test kits... -
Page 38
... to provide clinical laboratory testing services, including inaccurate testing results and other exposures. Our insurance coverage limits our maximum exposure on individual claims; however, we are essentially self-insured for a signiï¬cant portion of these claims. The basis for claims reserves... -
Page 39
...including clinical laboratories. These healthcare insurers, as well as independent physician associations, demand that clinical laboratory service providers accept discounted fee structures, or assume all or a portion of the ï¬nancial risk associated with providing testing services to their members... -
Page 40
... of New York. Quest Diagnostics and NID have been cooperating with the United States Attorney's Ofï¬ce. In connection with such cooperation, we have been providing information and producing various business records of NID and Quest Diagnostics, including documents related to testing and test kits... -
Page 41
...ï¬les to new data centers, presents signiï¬cant conversion risks that need to be managed carefully. In addition, public and private initiatives at the federal, state and regional levels to create HIT standards for the electronic exchange of clinical information, including laboratory results, could... -
Page 42
...healthcare providers. We consider a "payer'' to be the party that pays for the test and a "customer'' to be the party who refers the test to us. Depending on the billing arrangement and applicable law, we must bill various payers, such as patients, insurance companies, Medicare, Medicaid, physicians... -
Page 43
... or inaccurate billing information provided by ordering physicians; and • disputes with payers as to which party is responsible for payment. We incur additional costs as a result of our participation in Medicare and Medicaid programs, as billing and reimbursement for clinical laboratory testing is... -
Page 44
... insurance programs for claims that could result from providing or failing to provide clinical laboratory testing services, including inaccurate testing results and other exposures. Our insurance coverage limits our maximum exposure on individual claims; however, we are essentially self-insured... -
Page 45
...regulations restrict our ability to use or disclose patient-identiï¬able laboratory data, without patient authorization, for purposes other than payment, treatment or healthcare operations (as deï¬ned by HIPAA), except for disclosures for various public policy purposes and other permitted purposes... -
Page 46
... testing. Representatives of clinical laboratories (including Quest Diagnostics) and the American Clinical Laboratory Association (our industry trade association) have met with representatives of the FDA to address industry issues pertaining to potential FDA regulation of genetic testing in general... -
Page 47
... us from selling certain of our tests. Other companies or individuals, including our competitors, may obtain patents or other property rights that would prevent, limit or interfere with our ability to develop, perform or sell our tests or operate our business. As a result, we may be involved in... -
Page 48
... for Clinical Laboratory Services'' and "Business - Payers and Customers - Healthcare Insurers''. (d) The impact upon our testing volume and collected revenue or general or administrative expenses resulting from our compliance with Medicare and Medicaid administrative policies and requirements... -
Page 49
... bill the Medicare and Medicaid programs or other adverse regulatory actions by federal, state and local agencies. See "Business - Regulation of Clinical Laboratory Operations''. (i) (j) Changes in federal, state or local laws or regulations, including changes that result in new or increased federal... -
Page 50
... as a result of recent acquisitions. Location Leased or Owned Phoenix, Arizona Los Angeles, California (3) Sacramento, California San Diego, California San Jose, California San Juan Capistrano, California Denver, Colorado New Haven, Connecticut Washington, D.C. (Chantilly, Virginia) Miami, Florida... -
Page 51
... on any of our testing facilities, we could ï¬nd alternative space at competitive market rates and relocate our operations to such new location. Item 3. Legal Proceedings In addition to the investigations described in "Business - Government Investigations and Related Claims'', we are involved in... -
Page 52
... Stock, Related Stockholder Matters and Issuer Purchases of Equity Securities Our common stock is listed and traded on the New York Stock Exchange under the symbol "DGX''. The following table sets forth, for the periods indicated, the high and low sales price per share as reported on the New York... -
Page 53
... See Management's Discussion and Analysis of Financial Condition and Results of Operations. Item 8. Financial Statements and Supplementary Data See Item 15 (a) 1 and 2. Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure None. Item 9A. Controls and Procedures... -
Page 54
... (54) is Senior Vice President, Diagnostics Testing Services. Mr. Zewe oversees diagnostic testing operations company-wide, including physician, clinical trials and drugs of abuse testing, as well as the diagnostic instruments business. Mr. Zewe joined the Company in 1994 as General Manager of the... -
Page 55
... Data: Quarterly Operating Results (unaudited) ...2. Financial Statement Schedule: Item F-1 F-3 F-4 F-5 F-6 F-7 F-37 Page Schedule II - Valuation Accounts and Reserves ...3. Exhibits ï¬led as part of this report: See (b) below. (b) Exhibits ï¬led as part of this report: Exhibit Number... -
Page 56
...10.13 Form of Acceptance by National City Bank as successor Rights Agent under the Rights Agreement (ï¬led as an Exhibit to the Company's 2003 annual report on Form 10-K and incorporated herein by reference) Registration Rights Agreement dated October 31, 2005, among Quest Diagnostics Incorporated... -
Page 57
... 333-88330) and incorporated herein by reference) Form of Employees Stock Purchase Plan, as amended (ï¬led as an Exhibit to the Company's 2004 annual report on Form 10-K and incorporated herein by reference) Form of 1996 Employee Equity Participation Program, as amended (ï¬led as an Exhibit to the... -
Page 58
...) Form of Quest Diagnostics Incorporated Supplemental Executive Retirement Plan, effective December 14, 2004 (ï¬led as an exhibit to the Company's current report on Form 8-K (Date of report: December 14, 2004) and incorporated herein by reference) Form of Senior Management Incentive Plan (ï¬led... -
Page 59
... caused this report to be signed on its behalf by the undersigned, thereunto duly authorized. Quest Diagnostics Incorporated Chairman of the Board, President and Chief Executive Ofï¬cer Senior Vice President and Chief Financial Ofï¬cer Vice President, Corporate Controller and Chief Accounting Of... -
Page 60
... Company and management's discussion and analysis of ï¬nancial condition and results of operations included elsewhere in this Annual Report on Form 10-K. Year Ended December 31, 2004 2003(b) 2002(c) (in thousands, except per share data) 2005(a) 2001 Operations Data: Net revenues ...Amortization... -
Page 61
..., which were redeemed principally through a conversion into common shares as of January 18, 2005, and outstanding stock options and restricted common shares granted under our Amended and Restated Employee Long-Term Incentive Plan and our Amended and Restated Director Long-Term Incentive Plan. 44 -
Page 62
... costs as a result of our participation in Medicare and Medicaid programs, as billing and reimbursement for clinical laboratory testing is subject to considerable and complex federal and state regulations. Compliance with applicable laws and regulations, as well as internal compliance policies and... -
Page 63
... its health screening and risk assessment services to life insurance companies, as well as its clinical diagnostic testing services to healthcare providers and drugs-of-abuse testing to employers. LabOne, with 2004 revenues of $468 million, has 3,100 employees and principal laboratories in Lenexa... -
Page 64
... associated with clinical laboratory testing; • reserves for general and professional liability claims; • reserves for legal proceedings; and • accounting for and recoverability of goodwill. Revenues and accounts receivable associated with clinical laboratory testing The process for estimating... -
Page 65
...healthcare insurer is at risk and if so, would reserve accordingly. Government payers Payments for clinical laboratory services made by the government are based on fee schedules set by governmental authorities. Receivables due from government payers under the Medicare and Medicaid programs represent... -
Page 66
...to provide clinical laboratory testing services, including inaccurate testing results and other exposures. Our insurance coverage limits our maximum exposure on individual claims; however, we are essentially self-insured for a signiï¬cant portion of these claims. While the basis for claims reserves... -
Page 67
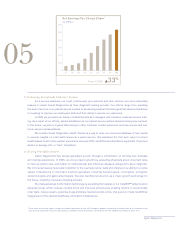
... from risk assessment services provided to life insurance companies, with the remainder classiï¬ed as clinical laboratory testing. Our clinical testing business, which accounted for over 95% of our 2005 consolidated net revenues, grew approximately 7.0% for the year. The acquisition of LabOne... -
Page 68
... enable healthcare providers to order and receive laboratory test results, order prescriptions electronically, and create, collect, manage and exchange healthcare information. Additionally, costs incurred at NID associated with completing its quality review and cooperating with an ongoing government... -
Page 69
... primarily relates to the improved collection of diagnosis, patient and insurance information necessary to more effectively bill for services performed. We believe that our Six Sigma and standardization initiatives and the increased use of electronic ordering by our customers will provide additional... -
Page 70
... of Unilab's results subsequent to February 28, 2003 served to reduce average revenue per requisition by 0.4% for the year ended December 31, 2004, reï¬,ecting Unilab's lower revenue per requisition. Drugs-of-abuse testing, which is among our lowest priced services and accounts for approximately... -
Page 71
... enable healthcare providers to order and receive laboratory test results, order prescriptions electronically, and create, collect, manage and exchange healthcare information. Cost of services, which includes the costs of obtaining, transporting and testing specimens, was 58.3% of net revenues for... -
Page 72
... Qualitative Disclosures About Market Risk We address our exposure to market risks, principally the market risk of changes in interest rates, through a controlled program of risk management that may include the use of derivative ï¬nancial instruments. In October 2005, we entered into interest rate... -
Page 73
... offset by the timing and net amount of various payments for taxes. Days sales outstanding, a measure of billing and collection efï¬ciency, improved to 46 days at December 31, 2005 from 47 days at December 31, 2004. Net cash provided by operating activities for 2004 was $799 million compared... -
Page 74
..., 2005, all of which was issued against the $85 million letter of credit lines. The letters of credit, which are renewed annually, primarily represent collateral for automobile liability and workers' compensation loss payments. Our credit agreements relating to our senior unsecured revolving credit... -
Page 75
... continue to improve and that over the long term the industry will continue to grow. As the leading provider of diagnostic testing, information and services with the most extensive network of laboratories and patient service centers throughout the United States, we believe we are well positioned to... -
Page 76
... ended December 31, 2005. Management's assessment of the effectiveness of the Company's internal control over ï¬nancial reporting as of December 31, 2005 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting ï¬rm, as stated in their report appearing on pages... -
Page 77
[THIS PAGE INTENTIONALLY LEFT BLANK] -
Page 78
...'s 2005 and 2004 consolidated ï¬nancial statements and of its internal control over ï¬nancial reporting as of December 31, 2005 and an audit of its 2003 consolidated ï¬nancial statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Our... -
Page 79
...it was acquired by the Company in a purchase business combination during 2005. We have also excluded LabOne, Inc. from our audit of internal control over ï¬nancial reporting. LabOne, Inc. is a whollyowned subsidiary whose total assets and total revenues represent 3.3% and 1.6%, respectively, of the... -
Page 80
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES CONSOLIDATED BALANCE SHEETS DECEMBER 31, 2005 AND 2004 (in thousands, except per share data) 2005 2004 Assets Current assets: Cash and cash equivalents ...Accounts receivable, net of allowance for doubtful accounts of $193,754 and $202,857 at ... -
Page 81
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF OPERATIONS FOR THE YEARS ENDED DECEMBER 31, 2005, 2004 AND 2003 (in thousands, except per share data) 2005 2004 2003 Net revenues ...Operating costs and expenses: Cost of services ...Selling, general and administrative ... -
Page 82
... with stock-based compensation plans ...Other, net ...Changes in operating assets and liabilities: Accounts receivable ...Accounts payable and accrued expenses ...Integration, settlement and other special charges ...Income taxes payable ...Other assets and liabilities, net ...Net cash provided by... -
Page 83
... to cover employee payroll tax withholdings on stock issued under beneï¬t plans ...Tax beneï¬ts associated with stockbased compensation plans ...Conversion of contingent convertible debentures ...Amortization of unearned compensation ...Purchases of treasury stock ...Balance, December 31, 2004... -
Page 84
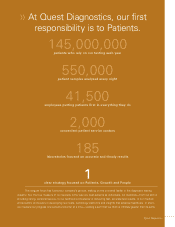
... life insurance industry. During 2005, Quest Diagnostics processed approximately 144 million requisitions through its extensive network of laboratories and patient service centers in virtually every major metropolitan area throughout the United States. 2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES... -
Page 85
... granted under the Company's Amended and Restated Employee Long-Term Incentive Plan and Amended and Restated Director Long-Term Incentive Plan. On June 20, 2005, the Company effected a two-for-one stock split through the issuance of a stock dividend of one new share of common stock for each share... -
Page 86
... requirements of SFAS 123 to require prominent disclosures in both annual and interim ï¬nancial statements about the method of accounting for stock-based employee compensation and the effect of the method used on reported results. The Company has chosen to adopt the disclosure only provisions of... -
Page 87
... to these customers, is limited. While the Company has receivables due from federal and state governmental agencies, the Company does not believe that such receivables represent a credit risk since the related healthcare programs are funded by federal and state governments, and payment is primarily... -
Page 88
... available information regarding comparable publicly-traded companies in the clinical laboratory testing industry, (ii) the ï¬nancial projections and future prospects of the Company's business, including its growth opportunities and likely operational improvements, and (iii) comparable sales prices... -
Page 89
...$56,467 Investments in available-for-sale equity securities consist primarily of equity securities in public corporations. Investments in trading equity securities represent participant directed investments of deferred employee compensation and related Company matching contributions held in a trust... -
Page 90
... deferred gains related to the settlement of certain treasury lock agreements (see Note 10). New Accounting Standards In December 2004, the FASB issued SFAS No. 123, revised 2004, "Share-Based Payment'' ("SFAS 123R''). SFAS 123R requires that companies recognize compensation cost relating to share... -
Page 91
... services for life insurance companies, its clinical diagnostic testing services, and its drugs-of-abuse testing for employers. LabOne has 3,100 employees and principal laboratories in Lenexa, Kansas, as well as in Cincinnati, Ohio. The acquisition of LabOne was accounted for under the purchase... -
Page 92
... the clinical laboratory testing industry, and further enhanced its national network and access to its comprehensive range of services for physicians, hospitals, patients and healthcare insurers. In connection with the acquisition of Unilab, as part of a settlement agreement with the United States... -
Page 93
... customers in the northern California area into its laboratories in San Jose and Sacramento. As of December 31, 2005, the Company operated two laboratories in the Los Angeles metropolitan area. As part of the integration plan, the Company plans to open a new regional laboratory in the Los Angeles... -
Page 94
... indicated) the collection and testing of specimens, as well as administrative and other support functions. Of the $9 million in costs, $7.9 million was recorded in the fourth quarter of 2003 and related to actions that impact the employees and operations of Unilab, was accounted for as a cost... -
Page 95
... payable at December 31, 2005 and 2004 were $29 million and $28 million, respectively, and consisted primarily of federal income taxes payable of $19 million and $25 million, respectively. The Company provides reserves for potential tax exposures that may arise from examinations by federal or state... -
Page 96
..., plant and equipment at December 31, 2005 and 2004 consisted of the following: 2005 2004 Land ...Buildings and improvements ...Laboratory equipment, furniture and ï¬xtures ...Leasehold improvements ...Computer software developed or obtained for internal use ...Construction-in-progress ...Less... -
Page 97
... the year ended December 31, 2004, the reduction in goodwill was primarily related to an increase in pre-acquisition tax net operating losses and credit carryforwards associated with businesses acquired. Amortizing intangible assets at December 31, 2005 and 2004 consisted of the following: Weighted... -
Page 98
... rate or federal funds rate. As of December 31, 2005 and 2004, the Company's borrowing rate for LIBOR-based loans was LIBOR plus 0.50% and 0.625%, respectively. The Credit Facility is guaranteed by the Company's wholly owned subsidiaries that operate clinical laboratories in the United States (the... -
Page 99
... is payable monthly at a rate adjusted weekly based on LIBOR plus approximately 0.08% or 4.5% as of December 31, 2005. The bonds are secured by the Lenexa, Kansas laboratory facility and an irrevocable bank letter of credit. Senior Notes In conjunction with its 2001 debt reï¬nancing, the Company... -
Page 100
... to those under the Credit Facility. Debentures due June 2034 In connection with the acquisition of LabOne, the Company assumed $103.5 million of 3.50% convertible senior debentures of LabOne due June 15, 2034 (the "Debentures due June 2034''). As a result of the change in control of LabOne, the... -
Page 101
...of Quest Diagnostics common stock trades with a preferred share purchase right, which entitles stockholders to purchase one-hundredth of a share of Series A Preferred Stock upon the occurrence of certain events. In conjunction with the SBCL acquisition, the Board of Directors of the Company approved... -
Page 102
.... 12. STOCK OWNERSHIP AND COMPENSATION PLANS Employee and Non-employee Directors Stock Ownership Programs In 2005, the Company established the Amended and Restated Employee Long-Term Incentive Plan (the "ELTIP'') to replace the Company's prior Employee Equity Participation Programs established in... -
Page 103
... the Amended and Restated Director Long-Term Incentive Plan (the "DLTIP''), to replace the Company's prior plan established in 1998. The DLTIP provides for the grant to nonemployee directors of non-qualiï¬ed stock options to purchase shares of Quest Diagnostics common stock at no less than... -
Page 104
...market price of the Company's common stock on the last business day of each calendar month. Under the ESPP, the maximum number of shares of Quest Diagnostics common stock which may be purchased by eligible employees is 8 million. The ESPP will terminate effective December 31, 2006. The Company plans... -
Page 105
...common stock for approximately $355 million from GSK. GSK has a long-term contractual relationship with Quest Diagnostics under which Quest Diagnostics is the primary provider of testing to support GSK's clinical trials testing requirements worldwide (the "Clinical Trials Agreements''). Net revenues... -
Page 106
... standing orders to purchase reagents and other laboratory supplies. At December 31, 2005, the approximate total future purchase commitments are $55 million, of which $28 million are expected to be incurred in 2006. In support of its risk management program, the Company has standby letters of credit... -
Page 107
... LabOne acquisition in 2005 (see Note 3), provides underwriting support services to the life insurance industry including teleunderwriting, specimen collection and paramedical examinations, laboratory testing, medical record retrieval, motor vehicle reports, telephone inspections and credit checks... -
Page 108
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED (dollars in thousands unless otherwise indicated) 2005 2004 2003 Net revenues: Clinical laboratory testing business ...All other operating segments ...Total net revenues ...Operating earnings (... -
Page 109
... the Company's Secured Receivables Credit Facility. The Company and the Subsidiary Guarantors provide collection services to QDRI. QDRI uses cash collections principally to purchase new receivables from the Company and the Subsidiary Guarantors. The following condensed consolidating ï¬nancial data... -
Page 110
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED (dollars in thousands unless otherwise indicated) Condensed Consolidating Balance Sheet December 31, 2005 Parent Subsidiary Guarantors NonGuarantor Subsidiaries Eliminations Consolidated Assets ... -
Page 111
... For the Year Ended December 31, 2005 Parent Subsidiary Guarantors NonGuarantor Subsidiaries Eliminations Consolidated Net revenues ...$874,113 $4,356,819 Operating costs and expenses: Cost of services ...491,029 2,572,377 Selling, general and administrative ...102,040 916,153 Amortization... -
Page 112
... 31, 2003 Parent Subsidiary Guarantors NonGuarantor Subsidiaries Eliminations Consolidated Net revenues ...$ 791,399 $3,709,590 Operating costs and expenses: Cost of services ...457,819 2,147,387 Selling, general and administrative ...76,626 880,951 Amortization of intangible assets ...1,723... -
Page 113
... net income to net cash provided by (used in) operating activities: Depreciation and amortization ...56,399 Provision for doubtful accounts ...4,940 Other, net ...(71,374) Changes in operating assets and liabilities ...163,057 Net cash provided by (used in) operating activities ...652,217 Net cash... -
Page 114
...35 (a) On November 1, 2005, Quest Diagnostics completed the acquisition of LabOne. The quarterly operating results include the results of operations of LabOne subsequent to the closing of the acquisition (see Note 3). (b) During the third quarter of 2005, the Company recorded a $6.2 million charge... -
Page 115
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES SCHEDULE II - VALUATION ACCOUNTS AND RESERVES (in thousands) Balance at 1-1-05 Provision for Doubtful Accounts Net Deductions and Other (a) Balance at 12-31-05 Year ended December 31, 2005 Doubtful accounts and allowances ... $202,857 $233,628 ... -
Page 116
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES RECONCILIATION OF NON-GAAP MEASURES The following is a reconciliation of non-GAAP measures presented in the ï¬nancial highlights to their most comparable measure under generally accepted accounting principles. 2005 Year ended December 31, 2004 2003 ... -
Page 117
...to฀our฀CEO฀and฀senior฀ financial฀officers฀will฀be฀posted฀on฀ our฀website. Quest฀Diagnostics฀has฀included฀as฀ Exhibit฀31฀to฀its฀Annual฀Report฀on฀ ฀ ฀ » Trademarks Quest฀Diagnostics,฀Quest,฀the฀฀ Sun-Q฀logo,฀ChartMaxx฀and฀Nichols... -
Page 118
...Business Quest฀Diagnostics฀is฀the฀leading฀provider฀of฀diagnostic฀testing,฀information฀and฀services฀that฀patients฀and฀doctors฀ need฀to฀make฀better฀healthcare฀decisions.฀The฀company฀offers฀the฀broadest฀access฀to฀diagnostic฀testing฀services...