Quest Diagnostics 2009 Annual Report Download - page 76
Download and view the complete annual report
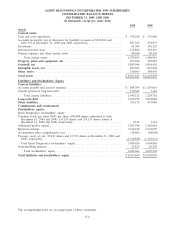
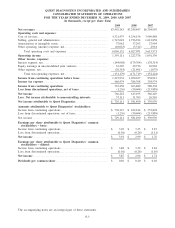
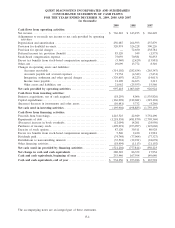
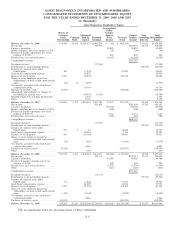
Please find page 76 of the 2009 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(dollars in thousands unless otherwise indicated)
1. DESCRIPTION OF BUSINESS
Quest Diagnostics Incorporated and its subsidiaries (“Quest Diagnostics” or the “Company”) is the world’s
leading provider of diagnostic testing, information and services, providing insights that enable patients, physicians
and others to make better healthcare decisions. Quest Diagnostics offers patients and physicians the broadest
access to diagnostic laboratory services through the Company’s nationwide network of laboratories and patient
service centers. The Company provides interpretive consultation through the largest medical and scientific staff in
the industry, with approximately 900 M.D.s and Ph.D.s primarily located in the United States. Quest Diagnostics
is the leading provider of clinical testing, including gene-based testing and other esoteric testing, anatomic
pathology services and testing for drugs-of-abuse, and the leading provider of risk assessment services for the life
insurance industry. The Company is also a leading provider of testing for clinical trials. The Company’s
diagnostics products business manufactures and markets diagnostic test kits and specialized point-of-care testing.
Quest Diagnostics empowers healthcare organizations and clinicians with state-of-the-art information technology
solutions that can improve patient care and medical practice.
During 2009, Quest Diagnostics processed approximately 148 million requisitions through its extensive
network of laboratories in virtually every major metropolitan area throughout the United States.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The consolidated financial statements include the accounts of all entities controlled by the Company through
its direct or indirect ownership of a majority voting interest and the accounts of any variable interest entities
where the Company is subject to a majority of the risk of loss from the variable interest entity’s activities, or
entitled to receive a majority of the entity’s residual returns or both. The Company’s relationships with variable
interest entities were not material at both December 31, 2009 and 2008. Investments in entities which the
Company does not control, but in which it has a substantial ownership interest (generally between 20% and 49%)
and can exercise significant influence, are accounted for using the equity method of accounting. As of December
31, 2009 and 2008, the Company’s investments in affiliates accounted for under the equity method of accounting
totaled $46.3 million and $38.4 million, respectively. The Company’s share of equity earnings from investments
in affiliates, accounted for under the equity method, totaled $33.2 million, $29.7 million and $27.0 million,
respectively, for 2009, 2008 and 2007. All significant intercompany accounts and transactions are eliminated in
consolidation.
Basis of Presentation
On January 1, 2009, the Company adopted a new accounting standard issued by the Financial Accounting
Standards Board (“FASB”) that establishes accounting and reporting standards for noncontrolling interests in a
subsidiary in consolidated financial statements. In accordance with this new standard, the Company has provided
a new presentation on the face of the consolidated financial statements to separately classify noncontrolling
interests within the equity section of the consolidated balance sheets and to separately report the amounts
attributable to controlling and noncontrolling interests in the consolidated statements of operations, comprehensive
income and changes in equity for all periods presented. The adoption of this standard did not impact earnings per
share attributable to Quest Diagnostics’ common stockholders.
In June 2009, the FASB issued the FASB Accounting Standards Codification (the “ASC”). The ASC has
become the single source of non-governmental accounting principles generally accepted in the United States
(“GAAP”) recognized by the FASB in the preparation of financial statements. The ASC does not supersede the
rules or regulations of the Securities and Exchange Commission (“SEC”), therefore, the rules and interpretive
releases of the SEC continue to be additional sources of GAAP for the Company. The Company adopted the
ASC as of July 1, 2009. The ASC does not change GAAP and did not have an effect on the Company’s
financial position, results of operations or cash flows.
During the third quarter of 2006, the Company completed its wind-down of NID, a test kit manufacturing
subsidiary, and classified the operations of NID as discontinued operations. The accompanying consolidated
statements of operations and related disclosures have been prepared to report the results of NID as discontinued
operations for all periods presented. See Note 16 for a further discussion of discontinued operations.
F-6