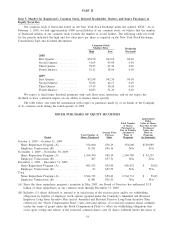
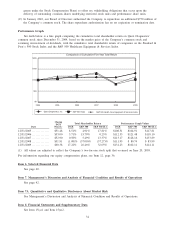
Quest Diagnostics 2009 Annual Report Download - page 36
Download and view the complete annual report
Please find page 36 of the 2009 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.The development of new, more cost-effective tests that can be performed by our customers or by patients,
or the internalization of testing by hospitals or physicians, could negatively impact our testing volume and
net revenues.
Advances in technology may lead to the development of more cost-effective tests that can be performed
outside of a commercial clinical laboratory such as (1) point-of-care tests that can be performed by physicians in
their offices, (2) esoteric tests that can be performed by hospitals in their own laboratories or (3) home testing
that can be performed by patients in their homes or by physicians in their offices. Although the CLIA
compliance costs make it cost prohibitive for many physicians to operate clinical laboratories in their offices,
manufacturers of laboratory equipment and test kits could seek to increase their sales by marketing point-of-care
test equipment to physicians. Diagnostic tests approved or cleared by the FDA for home use are automatically
deemed to be “waived” tests under CLIA and may be performed in physician office laboratories with minimal
regulatory oversight under CLIA as well as by patients in their homes. Test kit manufacturers could seek to
increase sales to both physicians and patients of test kits approved by the FDA for point-of-care testing or home
use. Development of such technology and its use by our customers would reduce the demand for our laboratory-
based testing services and negatively impact our net revenues.
Our customers, such as hospitals and physicians, may internalize tests that we currently perform. If our
customers were to internalize tests that we currently perform and we did not develop new or alternative tests
attractive to our customers, the demand for our testing services may be reduced and our net revenues may be
materially adversely impacted.
Our outstanding debt may impair our financial and operating flexibility.
As of December 31, 2009, we had approximately $3.1 billion of debt outstanding. Except for outstanding
letters of credit and operating leases, we do not have any off-balance sheet financing arrangements in place or
available. Our debt agreements contain various restrictive covenants. These restrictions could limit our ability to
use operating cash flow in other areas of our business because we must use a portion of these funds to make
principal and interest payments on our debt. We have obtained ratings on our debt from Standard and Poor’s and
Moody’s Investor Services and Fitch Ratings. There can be no assurance that any rating so assigned will remain
for any given period of time or that a rating will not be lowered or withdrawn entirely by a rating agency if in
that rating agency’s judgment future circumstances relating to the basis of the rating, such as adverse changes in
our Company or our industry, so warrant. If such ratings are lowered, the borrowing costs on our senior
unsecured revolving credit facility, secured receivables facility and term loan could increase. Changes in our
credit ratings, however, do not require repayment or acceleration of any of our debt.
We or our subsidiaries may incur additional indebtedness in the future. Our ability to make principal and
interest payments will depend on our ability to generate cash in the future. If we incur additional debt, a greater
portion of our cash flows may be needed to satisfy our debt service obligations and if we do not generate
sufficient cash to meet our debt service requirements, we may need to seek additional financing. In this case, it
may be more difficult, or we may be unable, to obtain financing on terms that are acceptable to us. As a result,
we would be more vulnerable to general adverse economic, industry and capital markets conditions as well as the
other risks associated with indebtedness.
Our ability to attract and retain qualified employees is critical to the success of our business and the
failure to do so may materially adversely affect our performance.
Our people are a critical resource. The supply of qualified personnel may be limited and competition for
qualified employees is strong. If we were to lose, or to fail to attract and retain, key management personnel or
qualified skilled technical or professional employees at our clinical laboratories, research centers or manufacturing
facilities, our earnings and revenues could be adversely affected. In addition, if we were to lose, or to fail to
attract and retain, skilled pathologists with positive relationships with their respective local medical communities,
particularly those with subspecialties, our earnings and revenues could be adversely affected.
Failure to establish, and perform to, appropriate quality standards to assure that the highest level of
quality is observed in the performance of our testing services and in the design, manufacture and
marketing of our products could adversely affect the results of our operations and adversely impact our
reputation.
The provision of clinical testing services, including anatomic pathology services, and related services, and
the design, manufacture and marketing of diagnostic products involve certain inherent risks. The services that we
26