Quest Diagnostics 2009 Annual Report Download - page 108
Download and view the complete annual report
Please find page 108 of the 2009 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
(j) Includes a benefit of $16.5 million primarily associated with favorable resolutions of certain tax
contingencies.
(k) Results for the years ended December 31, 2008 and 2007 reflect pre-tax charges of $75 million and $241
million, respectively, related to the government investigation of NID (see Note 16).
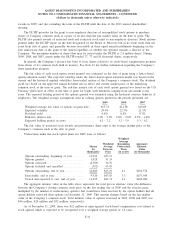
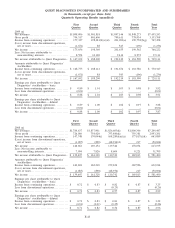
2009 2008 2007
Depreciation and amortization:
Clinical testing business. . ................................................... $200,905 $208,115 $189,939
All other operating segments ................................................ 17,337 18,414 19,301
General corporate........................................................... 38,445 38,064 28,639
Total depreciation and amortization .......................................... $256,687 $264,593 $237,879
Capital expenditures:
Clinical testing business. . ................................................... $136,248 $178,505 $193,785
All other operating segments ................................................ 23,592 22,891 17,760
General corporate........................................................... 7,088 11,285 7,556
Total capital expenditures ................................................... $166,928 $212,681 $219,101
18. SUBSEQUENT EVENTS
In January 2010, our Board of Directors authorized $750 million of additional share repurchases. The share
repurchase authorization has no set expiration or termination date.
Also, in January 2010, the Company executed an accelerated share repurchase transaction with a bank to
acquire approximately 4.5 million shares of the Company’s outstanding common stock, at an initial purchase
price of $56.05 per share, for $250 million. The purchase price for these shares is subject to an adjustment based
on the volume weighted average price of the Company’s common stock during a period following the execution
of the agreement.
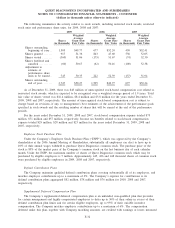
19. SUMMARIZED FINANCIAL INFORMATION
The Company’s Senior Notes due 2010, Senior Notes due 2011, Senior Notes due 2015, Senior Notes due
2017, Senior Notes due 2020, Senior Notes due 2037 and Senior Notes due 2040 are fully and unconditionally
guaranteed, jointly and severally, by the Subsidiary Guarantors. With the exception of Quest Diagnostics
Receivables Incorporated (see paragraph below), the non-guarantor subsidiaries are primarily foreign and less than
wholly-owned subsidiaries.
In conjunction with the Company’s Secured Receivables Credit Facility, the Company maintains a wholly-
owned non-guarantor subsidiary, Quest Diagnostics Receivables Incorporated (“QDRI”). The Company and certain
of its Subsidiary Guarantors transfer certain domestic receivables to QDRI. QDRI utilizes the transferred
receivables to collateralize borrowings under the Company’s Secured Receivables Credit Facility. The Company
and the Subsidiary Guarantors provide collection services to QDRI. QDRI uses cash collections principally to
purchase new receivables from the Company and the Subsidiary Guarantors.
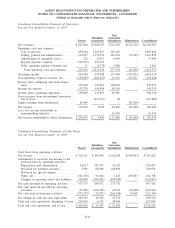
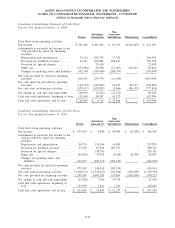
The following condensed consolidating financial data illustrates the composition of the combined guarantors.
Investments in subsidiaries are accounted for by the parent using the equity method for purposes of the
supplemental consolidating presentation. Earnings (losses) of subsidiaries are therefore reflected in the parent’s
investment accounts and earnings. The principal elimination entries relate to investments in subsidiaries and
intercompany balances and transactions.
F-38
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(dollars in thousands unless otherwise indicated)