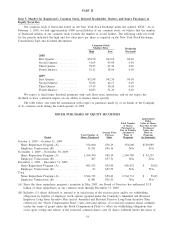
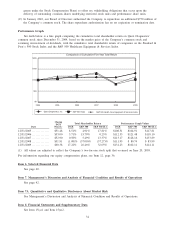
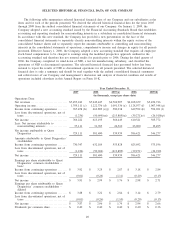
Quest Diagnostics 2009 Annual Report Download - page 40
Download and view the complete annual report
Please find page 40 of the 2009 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.(3) inability to obtain from patients a valid advance beneficiary notice form for tests that cannot be
billed without prior receipt of the form;
(4) increased challenges in operating as a non-contracted provider with respect to health plans; and
(5) the impact of additional or expanded limited coverage policies and limits on the allowable number
of test units.
(g) Adverse results from pending or future government investigations, lawsuits or private actions. These
include, in particular, monetary damages, loss or suspension of licenses, and/or suspension or exclusion
from the Medicare and Medicaid programs and/or criminal penalties.
(h) Failure to efficiently integrate acquired businesses and to manage the costs related to any such
integration, or to retain key technical, professional or management personnel.
(i) Denial, suspension or revocation of CLIA certification or other licenses for any of our clinical
laboratories under the CLIA standards, revocation or suspension of the right to bill the Medicare and
Medicaid programs or other adverse regulatory actions by federal, state and local agencies.
(j) Changes in federal, state or local laws or regulations, including changes that result in new or increased
federal or state regulation of commercial clinical laboratories or tests developed by commercial clinical
laboratories, including regulation of laboratory services by the FDA.
(k) Inability to achieve expected benefits from our acquisitions of other businesses.
(l) Inability to achieve additional benefits from our Six Sigma and efficiency initiatives.
(m) Adverse publicity and news coverage about the clinical testing industry or us.
(n) Computer or other IT system failures that affect our ability to perform tests, report test results or
properly bill customers, including potential failures resulting from the standardization of our IT systems
and other system conversions, telecommunications failures, malicious human acts (such as electronic
break-ins or computer viruses) or natural disasters.
(o) Development of technologies that substantially alter the practice of clinical test medicine, including
technology changes that lead to the development of more cost-effective tests such as (1) point-of-care
tests that can be performed by physicians in their offices, (2) esoteric tests that can be performed by
hospitals in their own laboratories or (3) home testing that can be carried out without requiring the
services of clinical laboratories.
(p) Negative developments regarding intellectual property and other property rights that could prevent, limit
or interfere with our ability to develop, perform or sell our tests or operate our business. These include:
(1) Issuance of patents or other property rights to our competitors or others; and
(2) Inability to obtain or maintain adequate patent or other proprietary rights for our products and
services or to successfully enforce our proprietary rights.
(q) Development of tests by our competitors or others which we may not be able to license, or usage of
our technology or similar technologies or our trade secrets by competitors, any of which could
negatively affect our competitive position.
(r) Regulatory delay or inability to commercialize newly developed or licensed products, tests or
technologies or to obtain appropriate reimbursements for such tests.
(s) Impact of any national healthcare information network or the adoption of standards for health
information technology interoperability that are incompatible with existing software and hardware
infrastructure requiring widespread replacement of systems and/or software.
(t) Inability to promptly or properly bill for our services or to obtain appropriate payments for services that
we do bill.
(u) Changes in interest rates and changes in our credit ratings from Standard & Poor’s, Moody’s Investor
Services or Fitch Ratings causing an unfavorable impact on our cost of and access to capital.
(v) Inability to hire and retain qualified personnel or the loss of the services of one or more of our key
senior management personnel.
(w) Terrorist and other criminal activities, hurricanes, earthquakes or other natural disasters, and health
pandemics, which could affect our customers, transportation or systems, or our facilities, and for which
insurance may not adequately reimburse us.
30