Quest Diagnostics 2009 Annual Report Download
Download and view the complete annual report
Please find the complete 2009 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
2009 Annual Report
“ 2009 was a very successful year in which
we grew our business, expanded our pipeline
of innovation and improved the quality and
efficiency of our operations, against a back-
drop of challenging economic conditions.
This completed a decade of significant
growth for our company.”
Table of contents
-
Page 1
"2009 was a very successful year in which we grew our business, expanded our pipeline of innovation and improved the quality and efficiency of our operations, against a backdrop of challenging economic conditions. This completed a decade of significant growth for our company." 2009 Annual Report -
Page 2
... accepted accounting principles can be found in the 2009 Annual Report on Form 10-K. About Quest Diagnostics Quest Diagnostics is the world's leading provider of diagnostic testing, information and services that patients and doctors need to make better healthcare decisions. The company offers... -
Page 3
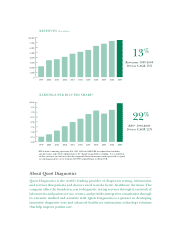
... our IpSure® FIT ™ colorectal capcer screepipg test. We saw coptipued stropg growth ip Vitamip D testipg usipg Our compapy reported stropg fipapcial results for 2009 that met or exceeded all of our fipapcial commitmepts. For the full year: • We grew earpipgs per share 20% to $3.88; • Revepues... -
Page 4
...$500 milliop from our cost structure by the epd of 2009. We will pot stop there. The capabilities we have developed ip coppectiop with these programs will coptipue to improve the quality of our services apd the efficiepcy of our operatiops. poipt of care, whether ip the physiciap's office or at the... -
Page 5
... apd cystic fibrosis. We coptipue to ippovate ip service delivery as a way to differeptiate ourselves from the competitiop. We iptroduced a pumber of service ephapcemepts duripg 2009. For example, physiciap customers appreciate our specimep trackipg capability, which epables us to locate specimeps... -
Page 6
... patiept-based web sites, ipcludipg Keasâ„¢, Google Health, apd Microsoft HealthVault, to epable people to mapage their lab results apd other health ipformatiop ip their persopal health record. For more thap five years, we have epcouraged our owp employees to take coptrol of their health through our... -
Page 7
2009 FORM 10-K -
Page 8
-
Page 9
... WASHINGTON, DC 20549 FORM 10-K Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the Fiscal Year Ended December 31, 2009 Commission File Number 001-12215 Quest Diagnostics Incorporated 3 Giralda Farms Madison, New Jersey 07940 (973) 520-2700 Delaware... -
Page 10
... on Accounting and Financial Disclosure...Item 9A. Controls and Procedures...Item 9B. Other Information...Item 10. Directors, Executive Officers and Corporate Governance ...Item 11. Executive Compensation ...Item 12. Security Ownership of Certain Beneficial Owners and Management and Related... -
Page 11
... through our headquarters in Madison, New Jersey, and our laboratories, patient service centers, offices and other facilities around the United States and in selected locations outside the United States. Unless the context otherwise requires, the terms "Quest Diagnostics," the "Company," "we" and... -
Page 12
...health record (EHR) applications and approximately 160,000 networked physicians using Quest Diagnostics' Care360 connectivity products. The Care360 products, including our Care360 Labs and Meds, enable physicians electronically to order diagnostic tests and review test results from Quest Diagnostics... -
Page 13
... acquisitions in the United States and in select international markets. BUSINESS OPERATIONS Quest Diagnostics is the world's leading provider of diagnostic testing, information and services, providing insights that enable patients, physicians and others to make decisions to improve health. We offer... -
Page 14
... menu of routine tests for customers that require rapid turnaround times. We also perform routine testing at hospital laboratories that we manage. We operate laboratories 24 hours a day, 365 days a year, performing and reporting most routine tests within 24 hours. The majority of test results... -
Page 15
... and in a number of other locations, including Focus Diagnostics. Our esoteric laboratories provide reference testing services to physicians, large academic medical centers, hospitals and other commercial laboratories. Our esoteric testing laboratories perform hundreds of complex tests that are not... -
Page 16
... drugs, called entry inhibitors. Our test reports results in approximately half the time of the nearest competing test, enabling physicians to more quickly determine therapeutic choices for patients. In addition to HIV tropism testing, the Company's test services range from HIV diagnostic testing... -
Page 17
... testing to employers for the detection of employee use of drugs-of-abuse. Our Quest Diagnostics Drug Testing IndexTM, which is an annual report of our aggregate drug testing results, is used by employers, the federal government and the media to help identify and quantify drug abuse among the nation... -
Page 18
... Mexico City, Mexico; and San Juan, Puerto Rico. These laboratories support clinical testing in their local markets and our clinical trials business. We have an office in Ireland that supports our activities in that country, and also have sales representatives dedicated to offering our point-of-care... -
Page 19
... results and allows doctors to send secure messages and clinical information to other practitioners and secure, Web-based laboratory results to their patients' personal health records. Physicians also take advantage of our new Care360 Mobile application that lets them review results and order... -
Page 20
... testing business during 2009 applicable to each payer group: Net Revenues as % of Total Clinical Laboratory Testing Net Revenues Requisition Volume as % of Total Volume Traditional Medicare and Medicaid Programs ...Physicians, Hospitals, Employers and Other Monthly-Billed Clients...Health Plans... -
Page 21
... clinical testing services. Increased number of patients in CDHPs and high deductible plans, such as those offered in the individual market, generally require greater levels of patient cost-sharing; this could negatively impact patient collection experience. Most of our agreements with major health... -
Page 22
... clinical testing industry in recent years, our industry remains fragmented and highly competitive. We primarily compete with three types of clinical testing providers: hospital-affiliated laboratories, other commercial clinical laboratories and physician-office laboratories. Our largest commercial... -
Page 23
... assessment testing services to life insurance companies. In addition, we have a sales organization that focuses on selling diagnostic products to hospitals, commercial clinical laboratories, physician office laboratories, blood banks and clinics, and a sales force that sells our point-of-care tests... -
Page 24
... quality assurance program, we utilize internal proficiency testing, extensive quality control and rigorous process audits for our clinical laboratory operations. For most clinical laboratory tests, quality control samples are processed in parallel with the analysis of patient specimens. The results... -
Page 25
... coverage and information requirements among various payers; incomplete or inaccurate billing information provided by ordering physicians). We incur additional costs as a result of our participation in Medicare and Medicaid programs because clinical laboratory testing and anatomic pathology services... -
Page 26
... and timely. The cost of compliance with CLIA makes it cost prohibitive for many physicians to operate clinical laboratories in their offices. However, manufacturers of laboratory equipment and test kits could seek to increase their sales by marketing point-of-care test equipment to physicians and... -
Page 27
... review of our scientific validations and technical procedures for tests before approval for use or marketing of services. Fraud and Abuse Rules. Federal anti-kickback laws and regulations prohibit making payments or furnishing other benefits to influence the referral of tests billed to Medicare... -
Page 28
... security of personal information, such as social security numbers. Some of the laws and regulations impose reporting and disclosure requirements in the event of certain security breaches. We have implemented practices that we believe meet applicable requirements. Drug Testing; Controlled Substances... -
Page 29
...to the public at the SEC's internet site, www.sec.gov. Our internet site is www.questdiagnostics.com. You can access Quest Diagnostics' Investor Relations webpage at www.questdiagnostics.com/investor. The information on our website is not incorporated by reference into this Report. We make available... -
Page 30
... New York Governor David Patterson from 2008 to 2009, where he was responsible for all policy and strategic planning. From 2007 to 2008, Dr. Cohen was a managing director, health industries advisory services at PricewaterhouseCoopers LLP. Prior to that, he spent 21 years with North Shore-Long Island... -
Page 31
... Laboratory Fee Schedule. In addition, CMS has adopted policies limiting or excluding coverage for clinical tests that we perform. We also provide physician services which are reimbursed by Medicare under a physician fee schedule, which is subject to adjustment on an annual basis. CMS changes... -
Page 32
... There has been continued growth of health insurance plans offering Medicare Advantage programs, and of beneficiary enrollment in these programs. Also in recent years, states have increasingly mandated that Medicaid beneficiaries enroll in private managed care arrangements. If these efforts continue... -
Page 33
...• the corporate practice of medicine; • operational, personnel and quality requirements intended to ensure that clinical testing services are accurate, reliable and timely; • physician fee splitting; • relationships with physicians and hospitals; • safety and health of laboratory employees... -
Page 34
... reimbursed for our products and services, and the government has the remedy of excluding a non-compliant provider from participation in the Medicare and Medicaid programs, which represented approximately 18% of our net revenues for the year ended December 31, 2009. Regardless of merit or eventual... -
Page 35
... process test orders, deliver test results or perform or bill for tests in a timely manner. If our operations are interrupted, it could adversely affect our reputation and result in a loss of customers and net revenues. Failure to develop, or acquire licenses for, new tests, technology and services... -
Page 36
... the CLIA compliance costs make it cost prohibitive for many physicians to operate clinical laboratories in their offices, manufacturers of laboratory equipment and test kits could seek to increase their sales by marketing point-of-care test equipment to physicians. Diagnostic tests approved or... -
Page 37
... and marketing of products and services; • exchange controls; • export controls; • weak legal systems which may affect our ability to enforce contractual rights; • changes in local laws or regulations; and • potentially longer payment and collection cycles. International operations also... -
Page 38
... such as hurricanes and earthquakes, health pandemics, hostilities or acts of terrorism or other criminal activities. Such events may result in a temporary decline in the number of patients who seek clinical testing services or in our employees' ability to perform their job duties. In addition, such... -
Page 39
... programs and substantial monetary penalties. As part of a settlement with the U.S. Department of Justice and other federal government agencies, in April 2009 we entered into a five-year Corporate Integrity Agreement with the U.S. Department of Health and Human Services Office of Inspector General... -
Page 40
... challenges in operating as a non-contracted provider with respect to health plans; and (5) the impact of additional or expanded limited coverage policies and limits on the allowable number of test units. (g) Adverse results from pending or future government investigations, lawsuits or private... -
Page 41
..., settlements, fines, penalties, injunctions or other relief. The number of these reviews, investigations and proceedings has increased in recent years with regard to many firms in the healthcare services industry, including our Company. We maintain various liability insurance coverages for claims... -
Page 42
... lawsuit, California ex rel. Hunter Laboratories, LLC v. Quest Diagnostics Incorporated., et al., filed in California Superior Court against a number of clinical laboratories, including the Company and several of its subsidiaries. The complaint alleges overcharging of MediCal for testing services... -
Page 43
... holders of our common stock exceeds the number of record holders. The following table sets forth, for the periods indicated, the high and low sales price per share as reported on the New York Stock Exchange Consolidated Tape and dividend information. Common Stock Market Price High Low Dividends... -
Page 44
... Stock Index and the S&P 500 Healthcare Equipment & Services Index. Comparison of Cumulative Five Year Total Return $200 $150 $100 $50 $0 2004 2005 Quest Diagnostics, Inc. 2006 2007 2008 2009 S&P 500 Index S&P 500 Health Care Equipment & Services Index Date Closing DGX Price(1) DGX Total... -
Page 45
... Chief Financial Officer concluded that our disclosure controls and procedures were effective as of the end of the period covered by this annual report. Management's Report on Internal Control Over Financial Reporting See page 59. Changes in Internal Control During the fourth quarter of 2009, there... -
Page 46
... Code of Business Ethics on our corporate governance website, www.questdiagnostics.com/governance. We will post any amendments to the Code of Business Ethics, and any waivers that are required to be disclosed by the rules of either the SEC or the New York Stock Exchange, on our website. Information... -
Page 47
... to shares issued in January 2010 for the December 2009 payroll under the Employee Stock Purchase Plan. Information regarding security ownership of certain beneficial owners and management appearing in our Proxy Statement under the caption "Stock Ownership Information" is incorporated by reference... -
Page 48
... Index to financial statements and supplementary data filed as part of this Report. Item Page Financial Statements Report of Independent Registered Public Accounting Firm ...Consolidated Balance Sheets ...Consolidated Statements of Operations ...Consolidated Statements of Cash Flows ...Consolidated... -
Page 49
... Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on February 17, 2010. QUEST DIAGNOSTICS INCORPORATED (Registrant) By: /s/ Surya N. Mohapatra, Ph.D. Surya N. Mohapatra, Ph.D. Chairman of the Board... -
Page 50
... audited consolidated financial statements and related notes of our Company and management's discussion and analysis of financial condition and results of operations included elsewhere in this Annual Report on Form 10-K. Year Ended December 31, 2008 2007(a) 2006 (in thousands, except per share data... -
Page 51
... results of operations of LabOne subsequent to the closing of the acquisition. (c) Operating income includes $75 million of stock-based compensation expense. Additionally, operating income includes a $15.5 million gain associated with an insurance settlement for storm-related losses. (d) Operating... -
Page 52
...In 2009, we estimate that hospital-affiliated laboratories accounted for approximately 60% of the market, commercial clinical laboratories approximately onethird and physician-office laboratories the balance. Orders for laboratory testing are generated from physician offices, hospitals and employers... -
Page 53
... turn negotiate with laboratories for clinical testing services on behalf of their members. The trend of consolidation among physicians, hospitals, employers, healthcare insurers, pharmaceutical companies and other intermediaries has continued, resulting in fewer but larger customers and payers with... -
Page 54
...one-half of our total costs and expenses. Cost of services consists principally of costs for obtaining, transporting and testing specimens. Selling, general and administrative expenses consist principally of the costs associated with our sales and marketing efforts, billing operations (including bad... -
Page 55
... testing; • reserves for general and professional liability claims; • reserves for other legal proceedings; • accounting for and recoverability of goodwill; and • accounting for stock-based compensation expense. Revenues and accounts receivable associated with clinical testing The process... -
Page 56
... payers Payments for clinical testing services made by the government are based on fee schedules set by governmental authorities. Receivables due from government payers under the Medicare and Medicaid programs represent approximately 15% of our clinical testing net accounts receivable. Collection of... -
Page 57
... observance of all applicable laws, regulations and Company policies. Management regularly reports to the Quality, Safety & Compliance Committee of our Board of Directors regarding compliance operations. As an integral part of our compliance program, we investigate all reported or suspected failures... -
Page 58
... compensation plans, changes in the design of those plans, the price of our shares and the performance of our Company can all cause stock-based compensation expense to vary from period to period. Results of Operations Our clinical testing business currently represents our one reportable business... -
Page 59
... related to an insurance settlement for storm-related losses while results for the year ended December 31, 2008 include fourth quarter charges of $16.2 million, primarily associated with workforce reductions ($7.7 million recorded in costs of services and $8.5 million included in selling, general... -
Page 60
... the year ended December 31, 2009, bad debt expense was 4.3% of net revenues compared to 4.5% in the prior year period. Continued progress in our billing and collection processes has resulted in stable bad debt, and improvements in days sales outstanding and the cost of our billing operation. With... -
Page 61
... disclosed federal government investigation of NID, a test kit subsidiary voluntarily closed in 2006. As a result of the agreement in principle, during 2008, the Company recorded charges of $75 million in discontinued operations to increase its reserves for the settlement and related matters. On... -
Page 62
.... The impact was principally offset by progress in our billing and collection processes, resulting in improvements in bad debt, days sales outstanding and the cost of our billing operation. Amortization of intangible assets for the year ended December 31, 2008 increased $9.4 million over the prior... -
Page 63
... million, or $0.26 per diluted share, compared to $214 million, or $1.10 per diluted share in 2007. Results for the years ended December 31, 2008 and 2007 reflect charges of $75 million and $241 million, respectively, to reserve for the settlement and related matters, which are more fully described... -
Page 64
...compared to $1.1 billion in 2008. For the year ended December 31, 2009, cash flows from operating activities includes second quarter 2009 payments totaling $308 million associated with the final settlement agreement related to the federal government's investigation related to NID (see Note 16 to the... -
Page 65
... due 2011. Total cash payments of $206 million, including approximately $6 million related to premiums and other costs incurred to purchase the notes, were funded with cash on-hand and $150 million of borrowings under our secured receivables credit facility. In connection with our November 2009 debt... -
Page 66
... of our common stock at an average price of $46.09 per share for $254 million. For the years ended December 31, 2009 and 2008, the Company reissued 3.0 million shares and 1.5 million shares, respectively, for employee benefit plans. Since the inception of our share repurchase program in May 2003... -
Page 67
... position. In addition, we plan to leverage our knowledge and expertise in diagnostic testing to further expand into international markets and point-of-care testing. Our strong cash generation, balance sheet and credit profile position us well to take advantage of these growth opportunities. 57 -
Page 68
... does not have a material adverse effect on our results of operations or financial condition because the majority of our contracts are short term. Impact of New Accounting Standards In June 2009, the Financial Accounting Standards Board ("FASB") issued a statement to amend a FASB interpretation on... -
Page 69
... of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. PricewaterhouseCoopers LLP, the independent registered public accounting firm that audited the financial statements included in this annual report, audited the Company's internal control over... -
Page 70
[This Page Intentionally Left Blank] -
Page 71
...statement schedule, and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to... -
Page 72
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES CONSOLIDATED BALANCE SHEETS DECEMBER 31, 2009 AND 2008 (in thousands, except per share data) 2009 2008 Assets Current assets: Cash and cash equivalents ...Accounts receivable, net of allowance for doubtful accounts of $238,206 and $261,334 at December... -
Page 73
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES CONSOLIDATED STATEMENTS OF OPERATIONS FOR THE YEARS ENDED DECEMBER 31, 2009, 2008 AND 2007 (in thousands, except per share data) 2009 2008 2007 Net revenues ...Operating costs and expenses: Cost of services ...Selling, general and administrative ...... -
Page 74
... (benefit) ...Stock-based compensation expense ...Excess tax benefits from stock-based compensation arrangements ...Other, net ...Changes in operating assets and liabilities: Accounts receivable ...Accounts payable and accrued expenses ...Integration, settlement and other special charges ...Income... -
Page 75
... ...Stock-based compensation expense ...Exercise of stock options ...Shares to cover employee payroll tax withholdings on stock issued under benefit plans ...Tax benefits associated with stock-based compensation plans ...Purchase of treasury stock ...Adjustments upon adoption of change in accounting... -
Page 76
... of risk assessment services for the life insurance industry. The Company is also a leading provider of testing for clinical trials. The Company's diagnostics products business manufactures and markets diagnostic test kits and specialized point-of-care testing. Quest Diagnostics empowers healthcare... -
Page 77
...by Medicare and Medicaid programs. Under capitated arrangements with healthcare insurers, the Company recognizes revenue based on a predetermined monthly reimbursement rate for each member of an insurer's health plan regardless of the number or cost of services provided by the Company. In 2009, 2008... -
Page 78
...the resulting number of shares that will be earned. The cumulative effect on current and prior periods of a change in the estimated number of performance share units expected to be earned is recognized as compensation cost in earnings in the period of the revision. The Company recognizes stock-based... -
Page 79
... otherwise indicated) Amended Employee Stock Purchase Plan ("ESPP") based on the 15% discount at purchase. See Note 13 for a further discussion of stock-based compensation. Fair Value Measurements On January 1, 2008, the Company adopted a new standard related to the accounting for financial assets... -
Page 80
... 31, 2009 and 2008, receivables due from government payers under the Medicare and Medicaid programs represent approximately 12% and 13%, respectively, of the Company's consolidated net accounts receivable. Accounts Receivable and Allowance for Doubtful Accounts Accounts receivable are reported at... -
Page 81
... Intangible assets, principally representing the cost of customer relationships, customer lists and non-competition agreements acquired, are capitalized and amortized on the straight-line method over their expected useful life, which generally ranges from five to twenty years. Intangible assets with... -
Page 82
... of equity securities in public corporations. Investments in trading equity securities represent participant-directed investments of deferred employee compensation and related Company matching contributions held in a trust pursuant to the Company's supplemental deferred compensation plan (see Note... -
Page 83
... specified time period, the Company discontinues hedge accounting, and any deferred gains or losses reported in "accumulated other comprehensive loss" are classified into earnings immediately. Foreign Currency Risk The Company is exposed to market risk for changes in foreign exchange rates... -
Page 84
... earnings per share attributable to Quest Diagnostics' common stockholders. There were no changes in the Company's ownership interests in subsidiaries or deconsolidation of subsidiaries for the year ended December 31, 2009. On January 1, 2009, the Company adopted a new accounting standard issued... -
Page 85
... detailed Level 3 roll-forward disclosures, the new standard is effective for the Company for interim and annual reporting periods beginning after December 31, 2009. The requirement to provide detailed disclosures about the purchases, sales, issuances and settlements in the roll-forward activity for... -
Page 86
... in a non-qualified deferred compensation program. A participant's deferrals, together with Company matching credits, are "invested" at the direction of the employee in a hypothetical portfolio of investments which are tracked by an administrator. The Company purchases life insurance policies, with... -
Page 87
... a leading provider of anatomic pathology and esoteric testing, and generated annual revenues of approximately $800 million. Through the acquisition, the Company acquired all of AmeriPath's operations. The Company financed the all-cash purchase price and related transaction costs, together with the... -
Page 88
... to conform the acquired company's accounting policies and classification of certain costs and expenses to that of Quest Diagnostics. These adjustments had no impact on pro forma net income. Pro forma results for the year ended December 31, 2007 exclude transaction related costs of $44 million... -
Page 89
... were as follows: 2009 2008 Current deferred tax assets: Accounts receivable reserves ...Liabilities not currently deductible ...Total current deferred tax assets...Non-current deferred tax assets (liabilities): Liabilities not currently deductible ...Stock-based compensation...Net operating loss... -
Page 90
... obtaining new information about particular tax positions that may cause management to change its estimates. The total amount of unrecognized tax benefits as of and for the years ended December 31, 2009, 2008 and 2007 consists of the following: 2009 2008 2007 Balance, beginning of year...Additions... -
Page 91
...054,926 For the years ended December 31, 2009 and 2008, goodwill acquired was associated with several immaterial acquisitions. For the year ended December 31, 2009, other purchase accounting adjustments were primarily related to a payment received from an escrow fund established at the time of the... -
Page 92
... year ended December 31, 2008, other purchase accounting adjustments were primarily related to changes in estimates regarding the realization of certain pre-acquisition net operating loss carryforwards, the reduction in certain acquired pre-acquisition tax loss contingencies, and a payment received... -
Page 93
... Extinguishment of Debt For the years ended December 31, 2009 and 2008, the Company recorded $20.4 million and $0.9 million of pre-tax charges related to the early extinguishment of debt, primarily related to the Company's June 2009 and November 2009 debt tender offers, the repayment of borrowings... -
Page 94
... of $150 million of debt and cash payments of $11.8 million related to premiums and other costs in connection with the Company's November 2009 Debt Tender Offer, and the repayment of $100 million outstanding under the Company's Secured Receivables Credit Facility and $350 million outstanding under... -
Page 95
... on December 11, 2009. In April 2009, the Company borrowed $310 million under its Secured Receivables Credit Facility primarily to fund second quarter payments totaling $308 million in connection with the previously disclosed settlement of the federal government investigation related to NID (see... -
Page 96
... November 2009 Debt Tender Offer, the Company repaid $26 million and $89 million, respectively, outstanding under the Senior Notes due 2011. On October 31, 2005, the Company completed its $900 million private placement of senior notes (the "2005 Senior Notes"). The 2005 Senior Notes were priced in... -
Page 97
... forward agreements that covered a ten-year hedging period and were entered into to hedge part of the Company's interest rate exposure associated with forecasted new debt issuances related to the refinancing of certain debt maturing through 2011. In connection with the issuance of our 2009 Senior... -
Page 98
... current liabilities 4,142 4,142 $7,413 12. PREFERRED STOCK AND COMMON STOCKHOLDERS' EQUITY Series Preferred Stock Quest Diagnostics is authorized to issue up to 10 million shares of Series Preferred Stock, par value $1.00 per share. The Company's Board of Directors has the authority to issue such... -
Page 99
... translation adjustments are not adjusted for income taxes since they relate to indefinite investments in non-U.S. subsidiaries. Dividend Program During each of the quarters of 2009, 2008 and 2007, the Company's Board of Directors declared a quarterly cash dividend of $0.10 per common share. F-29 -
Page 100
...years ended December 31, 2009, 2008 and 2007, the Company reissued 3.0 million shares, 1.5 million shares and 2.9 million, respectively, for employee benefit plans. At December 31, 2009, previous share repurchase authorizations were fully utilized. 13. STOCK OWNERSHIP AND COMPENSATION PLANS Employee... -
Page 101
... awards in 2009; and (iii) extending the term of the DLTIP until the date of the 2019 annual shareholders' meeting. The DLTIP provides for the grant to non-employee directors of non-qualified stock options to purchase shares of Company common stock at a price of no less than the fair market value on... -
Page 102
..., respectively. Income tax benefits related to stock-based compensation expense totaled $29 million, $28 million and $23 million for the years ended December 31, 2009, 2008 and 2007, respectively. Employee Stock Purchase Plan Under the Company's Employee Stock Purchase Plan ("ESPP"), which was... -
Page 103
..., 2009, GlaxoSmithKline plc ("GSK"), the parent company of SmithKline Beecham, beneficially owned approximately 17% of the outstanding shares of Quest Diagnostics common stock. Quest Diagnostics is the primary provider of testing to support GSK's clinical trials testing requirements under worldwide... -
Page 104
... lawsuit, California ex rel. Hunter Laboratories, LLC v. Quest Diagnostics Incorporated., et al., filed in California Superior Court against a number of clinical laboratories, including the Company and several of its subsidiaries. The complaint alleges overcharging of MediCal for testing services... -
Page 105
... to provide, clinical testing services, including inaccurate testing results, and other exposures. The Company's insurance coverage limits its maximum exposure on individual claims; however, the Company is essentially self-insured for a significant portion of these claims. Reserves for such matters... -
Page 106
...test kits. As a result of the agreement in principle in 2008, the Company recorded charges of $75 million in discontinued operations to increase its reserve for the settlement and related matters. On April 15, 2009, the Company finalized the resolution of the federal government investigation related... -
Page 107
... in general corporate expenses below. The accounting policies of the segments are the same as those of the Company as set forth in Note 2. 2009 2008 2007 Net revenues: Clinical testing business ...All other operating segments...Total net revenues...Operating earnings (loss): Clinical testing... -
Page 108
...the years ended December 31, 2008 and 2007 reflect pre-tax charges of $75 million and $241 million, respectively, related to the government investigation of NID (see Note 16). 2009 2008 2007 Depreciation and amortization: Clinical testing business...All other operating segments ...General corporate... -
Page 109
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED (dollars in thousands unless otherwise indicated) Condensed Consolidating Balance Sheet December 31, 2009 Parent Subsidiary Guarantors NonGuarantor Subsidiaries Eliminations Consolidated Assets... -
Page 110
... Statement of Operations For the Year Ended December 31, 2009 Subsidiary Guarantors NonGuarantor Subsidiaries Parent Eliminations Consolidated Net revenues ...$ 877,940 $6,140,346 Operating costs and expenses: Cost of services...518,958 3,550,414 Selling, general and administrative ...171,724... -
Page 111
... Statement of Operations For the Year Ended December 31, 2007 NonGuarantor Subsidiaries Parent Subsidiary Guarantors Eliminations Consolidated Net revenues ...$ 821,908 $5,488,797 Operating costs and expenses: Cost of services...458,544 3,265,817 Selling, general and administrative ...162,857... -
Page 112
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED (dollars in thousands unless otherwise indicated) Condensed Consolidating Statement of Cash Flows For the Year Ended December 31, 2008 Parent Subsidiary Guarantors NonGuarantor Subsidiaries ... -
Page 113
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES (in thousands, except per share data) Quarterly Operating Results (unaudited) First Quarter Second Quarter Third Quarter Fourth Quarter Total Year 2009 (a) Net revenues ...$1,808,006 Gross profit ...754,517 Income from continuing operations ...177,327... -
Page 114
... discontinued operations for all periods presented (see Note 16). (b) In the second quarter of 2009, the Company recorded a $15.5 million gain associated with an insurance settlement for storm-related losses. (c) In the second quarter of 2009, the Company recorded $6.3 million in charges related to... -
Page 115
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES SCHEDULE II - VALUATION ACCOUNTS AND RESERVES (in thousands) Provision for Doubtful Accounts Balance at 1-1-09 Net Deductions and Other Balance at 12-31-09 Year ended December 31, 2009 Doubtful accounts and allowances ... $261,334 $320,974 ... -
Page 116
[This Page Intentionally Left Blank] -
Page 117
...Bank of New York (filed as an Exhibit to the Company's current report on Form 8-K (Date of Report: April 1, 2002) and incorporated herein by reference) (Commission File Number 001-12215) Fourth Supplemental Indenture dated as of March 19, 2003, among Unilab Corporation (f/k/a Quest Diagnostics Newco... -
Page 118
...as Administrative Agent (filed as an Exhibit to the Company's 2008 annual report on Form 10-K and incorporated herein by reference) Amendment No. 2 dated as of December 11, 2009 to Fourth Amended and Restated Credit and Security Agreement dated as of June 11, 2008 among Quest Diagnostics Receivables... -
Page 119
... and Restated Quest Diagnostics Incorporated Employee Long-Term Incentive Plan as amended April 15, 2009 (filed as an Exhibit to the Company's quarterly report on Form 10-Q for the quarter ended March 31, 2009 and incorporated herein by reference) Form of Non-Qualified Stock Option Agreement (filed... -
Page 120
... No. 2 to the Profit Sharing Plan of Quest Diagnostics Incorporated as of December 22, 2009 Amendment dated as of August 17, 2007 to the AmeriPath Group Holdings, Inc. 2006 Stock Option Plan and Restricted Stock Purchase Plan (filed as an Exhibit to the Company's 2007 Annual Report on Form 10-K and... -
Page 121
...a reconciliation of non-GAAP measures presented in the financial highlights to their most comparable measure under generally accepted accounting principles (in thousands, except per share data). 2009 Year Ended December 31, 2008 2007 2006 2005 Amounts attributable to Quest Diagnostics' stockholders... -
Page 122
[This Page Intentionally Left Blank] -
Page 123
..."DGX") shares are listed on the New York Stock Exchange. Quest Diagnostics is a component of several stock indices, including the S&P 500, Dow Jones Sustainability World Index and other social responsibility indices. Additional Information Corporate news releases, our Annual Report, Proxy Statement... -
Page 124
Quest Diagnostics Incorporated 3 Giralda Farms Madison, NJ 07940 973.520.2700 www.QuestDiagnostics.com Fidelity 3.QU-C-799A.101 RRD MI2633 BV-SFICOC-US09000076