Quest Diagnostics 2011 Annual Report Download - page 39
Download and view the complete annual report
Please find page 39 of the 2011 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Item 1B. Unresolved Staff Comments
There are no unresolved SEC comments that require disclosure.
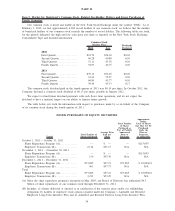
Item 2. Properties
Our executive offices are located in Madison, New Jersey. We maintain clinical testing laboratories in major
metropolitan areas and elsewhere throughout the continental United States; in several instances a joint venture of
which we are a partner maintains the laboratory. We also maintain offices, data centers, billing centers, call
centers, an assembly center, distribution centers, patient service centers and a clinical trials testing laboratory at
locations throughout the United States. In addition, we maintain offices, manufacturing facilities, patient service
centers and clinical laboratories in locations outside the United States, including in Sweden, Puerto Rico, Mexico,
the United Kingdom, India, Ireland and Australia. Our properties that are not owned are leased on terms and for
durations that are reflective of commercial standards in the communities where these properties are located. We
believe that, in general, our facilities are suitable and adequate for our current and anticipated future levels of
operation and are adequately maintained. We believe that if we were unable to renew a lease on any of our
facilities, we could find alternative space at competitive market rates and relocate our operations to such new
location without material disruption to our business. Several of our principal facilities are highlighted below.
Location Leased or Owned
Cypress, California (laboratory) . . ..................... Leased
West Hills, California (laboratory)..................... Leased
San Juan Capistrano, California (laboratory) ........... Owned
Tampa, Florida (laboratory) ........................... Owned
Atlanta, Georgia (laboratory) .......................... Owned
Chicago, Illinois (2) (laboratories) ..................... One owned, one leased
Baltimore, Maryland (laboratory) . ..................... Owned
Teterboro, New Jersey (laboratory) .................... Owned
Philadelphia, Pennsylvania (laboratory) ................ Leased
Norristown, Pennsylvania (offices)..................... Leased
Dallas, Texas (laboratory)............................. Leased
Chantilly, Virginia (laboratory) . . . ..................... Leased
Item 3. Legal Proceedings
In addition to the matters described below, in the normal course of business, we have been named, from
time to time, as a defendant in various legal actions, including arbitrations, class actions and other litigation,
arising in connection with our activities as a provider of diagnostic testing, information and services. These legal
actions may include lawsuits alleging negligence or other similar legal claims. These actions could involve claims
for substantial compensatory and/or punitive damages or claims for indeterminate amounts of damages, and could
have an adverse impact on our client base and reputation.
We are also involved, from time to time, in other reviews, investigations and proceedings by governmental
agencies regarding our business, including, among other matters, operational matters, which may result in adverse
judgments, settlements, fines, penalties, injunctions or other relief. The number of these reviews, investigations
and proceedings has increased in recent years with regard to many firms in the healthcare services industry,
including our Company.
Legal Matters
The Company is involved in various legal proceedings. Some of the proceedings against the Company
involve claims that could be substantial in amount.
In November 2009, the U.S. District Court for the Southern District of New York partially unsealed a civil
complaint, U.S. ex rel. Fair Laboratory Practices Associates v. Quest Diagnostics Incorporated, filed against the
Company under the whistleblower provisions of the federal False Claims Act. The complaint alleged, among
other things, violations of the federal Anti-Kickback Statute and the federal False Claims Act in connection with
the Company’s pricing of laboratory services. The complaint seeks damages for alleged false claims associated
with laboratory tests reimbursed by government payors, treble damages and civil penalties. In March 2011, the
33