Quest Diagnostics 2011 Annual Report Download - page 108
Download and view the complete annual report
Please find page 108 of the 2011 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
management does not anticipate that the ultimate outcome of any such proceedings or claims will have a material
adverse effect on the Company’s financial condition, given the high degree of judgment involved in establishing
accruals for loss estimates related to these types of matters, the outcome may be material to the Company’s
results of operations or cash flows in the period in which the impact of such claims is determined or paid.
17. DISCONTINUED OPERATIONS
During the third quarter of 2006, the Company completed its wind down of NID, a test kit manufacturing
subsidiary, and classified the operations of NID as discontinued operations. Results of operations for NID have
been reported as discontinued operations in the accompanying consolidated statements of operations and related
disclosures for all periods presented.
On April 15, 2009, the Company finalized the resolution of the federal government investigation related to
NID and entered into a final settlement agreement with the federal government. In the second quarter of 2009,
the Company paid $268 million to settle the civil allegations. The Company also entered into a five-year
corporate integrity agreement with the Office of Inspector General for the United States Department of Health
and Human Services. In addition, NID pled guilty to a single count of felony misbranding and paid a $40 million
fine. These payments totaling $308 million, which had been previously reserved, were funded out of cash on-
hand and available credit facilities. During the third quarter of 2009, the Company finalized separate settlement
agreements with certain states and paid approximately $6 million, which had been previously reserved for.
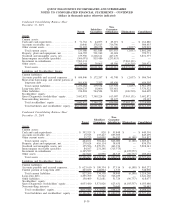
Summarized financial information for the discontinued operations of NID is set forth below:
2011 2010 2009
Net revenues ......................................................... $ — $ — $ —
Loss from discontinued operations before income taxes................. (366) (22) (2,361)
Income tax (expense) benefit. . . ....................................... (1,216) (1,765) 1,125
Loss from discontinued operations, net of taxes . . . ..................... $(1,582) $(1,787) $(1,236)
The remaining balance sheet information related to NID was not material at December 31, 2011 and 2010.
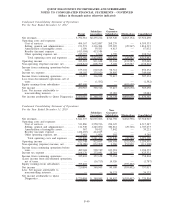
18. BUSINESS SEGMENT INFORMATION
Clinical testing is an essential element in the delivery of healthcare services. Physicians use clinical tests to
assist in the detection, diagnosis, evaluation, monitoring and treatment of diseases and other medical conditions.
Clinical testing is generally categorized as clinical laboratory testing and anatomic pathology services. Clinical
laboratory testing is generally performed on whole blood, serum, plasma and other body fluids, such as urine, and
specimens such as microbiology samples. Anatomic pathology services are principally for the detection of cancer
and are performed on tissues, such as biopsies, and other samples, such as human cells. Customers of the clinical
testing business include patients, physicians, hospitals, employers, governmental institutions and other commercial
clinical laboratories. The clinical testing business accounted for greater than 90% of net revenues from continuing
operations in 2011, 2010 and 2009.
All other operating segments include the Company’s non-clinical testing businesses and consist of its risk
assessment services, clinical trials testing, healthcare information technology, and diagnostics products businesses.
The Company’s risk assessment services business provides underwriting support services to the life insurance
industry including electronic data collection, specimen collection and paramedical examinations, laboratory testing,
medical record retrieval, case management, motor vehicle reports, telephone inspections, prescription histories and
credit checks. The Company’s clinical trials testing business provides clinical testing performed in connection
with clinical research trials on new drugs, vaccines and certain medical devices. The Company’s healthcare
information technology business is a developer and integrator of clinical connectivity and data management
solutions for healthcare organizations, physicians and clinicians that can help improve patient care and medical
practice. The Company’s diagnostics products business manufactures and markets products that enable healthcare
professionals to make healthcare diagnoses, including products for point-of-care, or near-patient, testing for the
professional market. During the second quarter of 2011, the Company acquired Athena and Celera (see Note 4
for further details). Athena is included in the Company’s clinical laboratory testing business. The majority of
F-36
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(dollars in thousands unless otherwise indicated)