Quest Diagnostics 2006 Annual Report Download - page 120
Download and view the complete annual report
Please find page 120 of the 2006 Quest Diagnostics annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
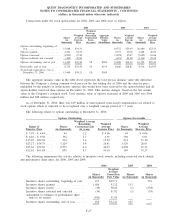
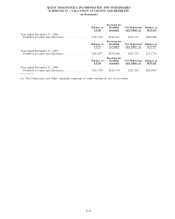
2006 2005 2004
Depreciation and amortization:
Clinical laboratory testing business .......................................... $167,672 $156,920 $148,804
All other operating segments ................................................ 16,375 8,441 6,919
General corporate........................................................... 11,640 5,822 7,610
Discontinued operations . . ................................................... 1,711 4,941 5,393
Total depreciation and amortization .......................................... $197,398 $176,124 $168,726
Capital expenditures:
Clinical laboratory testing business .......................................... $168,636 $204,469 $167,203
All other operating segments ................................................ 17,291 13,445 3,657
General corporate........................................................... 6,722 3,912 2,379
Discontinued operations . . ................................................... 773 2,444 2,886
Total capital expenditures ................................................... $193,422 $224,270 $176,125
17. SUBSEQUENT EVENT
Acquisition of HemoCue
On January 31, 2007, the Company acquired POCT Holding AB (“HemoCue”), a Sweden-based company
specializing in point-of-care testing, also referred to as near patient testing, in an all-cash transaction valued at
approximately $420 million, including $123 million of assumed debt of HemoCue. The transaction, which has
been financed through a new credit facility, is not expected to have a material impact on the Company’s 2007
financial results.
HemoCue is the leading international provider in near patient testing for hemoglobin, with a growing share
in professional glucose and microalbumin testing. In addition, HemoCue is currently developing new tests
including a near patient test to determine white blood cell counts.
New Credit Facility
On January 31, 2007, the Company entered into an Interim Credit Agreement (“Interim Credit Facility”) for
a $450 million senior unsecured loan and borrowed $450 million to acquire HemoCue, and to pay fees, costs and
expenses incurred in connection with the acquisition.
Under the Interim Credit Facility, which matures on January 31, 2008, interest is based on certain published
rates plus an applicable margin that will vary over an approximate range of 45 basis points based on changes in
the Company’s public debt rating. At its option, the Company may elect to enter into LIBOR-based interest rate
contracts for periods up to six months. Interest on any outstanding amounts not covered under the LIBOR-based
interest rate contracts is based on an alternate base rate, which is calculated by reference to the prime rate or
federal funds rate. The Interim Credit Facility is guaranteed by the Company’s domestic wholly owned operating
subsidiaries. The Interim Credit Facility contains various covenants similar to those under the Credit Facility. In
addition, the Interim Credit Facility provides for the mandatory pre-payment of the loan in the event of a debt or
equity issuance by the Company, subject to certain limited exceptions as set forth in the Interim Credit
Agreement.
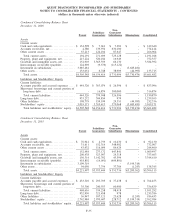
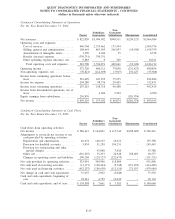
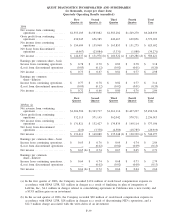
18. SUMMARIZED FINANCIAL INFORMATION
As described in Note 10, the 2005 Senior Notes, the 2001 Senior Notes and the Debentures are fully and
unconditionally guaranteed by the Subsidiary Guarantors. With the exception of Quest Diagnostics Receivables
Incorporated (see paragraph below), the non-guarantor subsidiaries are primarily foreign and less than wholly
owned subsidiaries. In January 2005, the Company completed its redemption of all of its outstanding Debentures.
In July 2006, the Company repaid at maturity the $275 million outstanding under its Senior Notes due 2006.
In conjunction with the Company’s Secured Receivables Credit Facility described in Note 10, the Company
maintains a wholly owned non-guarantor subsidiary, Quest Diagnostics Receivables Incorporated (“QDRI”). The
F-33
QUEST DIAGNOSTICS INCORPORATED AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS - CONTINUED
(dollars in thousands unless otherwise indicated)