Pfizer 2011 Annual Report Download - page 84
Download and view the complete annual report
Please find page 84 of the 2011 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
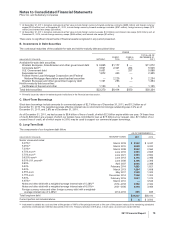
B. Other Intangible Assets
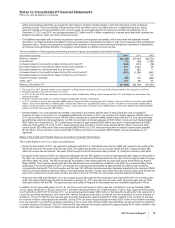
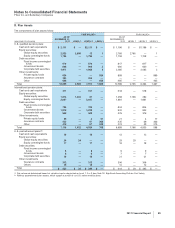
The components of identifiable intangible assets follow:
AS OF DECEMBER 31,
2011 2010
(MILLIONS OF DOLLARS)
GROSS
CARRYING
AMOUNT
ACCUMULATED
AMORTIZATION
IDENTIFIABLE
INTANGIBLE
ASSETS, LESS
ACCUMULATED
AMORTIZATION
GROSS
CARRYING
AMOUNT
ACCUMULATED
AMORTIZATION
IDENTIFIABLE
INTANGIBLE
ASSETS, LESS
ACCUMULATED
AMORTIZATION
Finite-lived intangible assets:
Developed technology rights(a) $73,088 $(32,013) $41,075 $68,432 $(26,223) $42,209
Brands 1,678 (687) 991 1,626 (607) 1,019
License agreements 425 (215) 210 637 (248) 389
Other 623 (362) 261 533 (324) 209
Total finite-lived intangible assets 75,814 (33,277) 42,537 71,228 (27,402) 43,826
Indefinite-lived intangible assets:
Brands 10,027 — 10,027 10,219 — 10,219
In-process research and development(a) 1,197 — 1,197 3,438 — 3,438
Trademarks 72 — 72 72 — 72
Total indefinite-lived intangible assets 11,296 — 11,296 13,729 — 13,729
Total identifiable intangible assets(b) $87,110 $(33,277) $53,833 $84,957 $(27,402) $57,555
(a) In the fourth quarter of 2011, Prevenar 13 Adult and Vyndaqel (tafamidis meglumine) received regulatory approval in a major market, and as a
result, we reclassified these assets, with a combined book value of approximately $2.3 billion, from IPR&D to Developed Technology Rights and
began to amortize the assets.
(b) The decrease is primarily related to amortization and impairment charges (see Note 4. Other Deductions—Net), partially offset by assets acquired
as part of the acquisition of King (see Note 2B. Acquisitions, Divestitures, Collaborative Arrangements and Equity-Method Investments: Acquisition
of King Pharmaceuticals, Inc.) and the impact of foreign exchange.
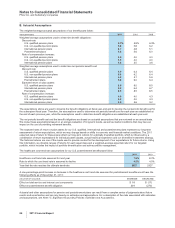
At December 31, 2011, our identifiable intangible assets are associated with the following, as a percentage of identifiable intangible
assets, less accumulated amortization:
•Developed technology rights: Specialty Care (64%); Established Products (17%); Primary Care (15%); Animal Health (2%); Oncology
(1%); and Nutrition (1%)
•Brands, finite-lived: Consumer Healthcare (57%); Established Products (29%); and Animal Health (14%)
•Brands, indefinite-lived: Consumer Healthcare (51%); Established Products (26%); and Nutrition (23%)
•IPR&D: Worldwide Research and Development (57%); Specialty Care (14%); Primary Care (14%); Established Products (8%);
Oncology (5%); and Animal Health (2%)
There are no percentages for our Emerging Markets business unit as it is a geographic-area unit, not a product-based unit. The
carrying value of the assets associated with our Emerging Markets business unit is included within the assets associated with the
other four biopharmaceutical business units.
For information about intangible asset impairments, see Note 4. Other Deductions––Net.
Developed Technology Rights
Developed technology rights represent the amortized cost associated with developed technology, which has been acquired from
third parties and which can include the right to develop, use, market, sell and/or offer for sale the product, compounds and
intellectual property that we have acquired with respect to products, compounds and/or processes that have been completed. We
possess a well-diversified portfolio of hundreds of developed technology rights across therapeutic categories, primarily representing
the commercialized products included in our five biopharmaceutical business units. Virtually all of these assets were acquired in
connection with our Wyeth acquisition in 2009 and our Pharmacia acquisition in 2003. The more significant components of
2011 Financial Report 83