Pfizer 2011 Annual Report Download - page 102
Download and view the complete annual report
Please find page 102 of the 2011 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
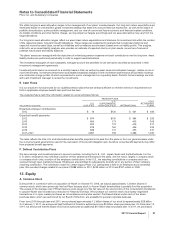
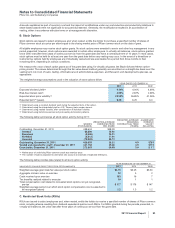
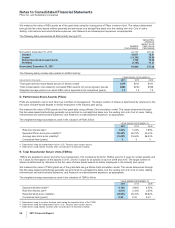
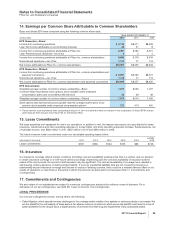
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
Numerous lawsuits are pending against Pfizer in various federal and state courts seeking damages for alleged personal injury from
exposure to products containing asbestos and other allegedly hazardous materials sold by Gibsonburg Lime Products Company
(Gibsonburg). Gibsonburg was acquired by Pfizer in the 1960s and sold products containing small amounts of asbestos until the
early 1970s.
There also is a small number of lawsuits pending in various federal and state courts seeking damages for alleged exposure to
asbestos in facilities owned or formerly owned by Pfizer or its subsidiaries.
Celebrex and Bextra
•Securities and ERISA Actions
Beginning in late 2004, actions, including purported class actions, were filed in various federal and state courts against Pfizer,
Pharmacia Corporation (Pharmacia) and certain current and former officers, directors and employees of Pfizer and Pharmacia.
These actions include (i) purported class actions alleging that Pfizer and certain current and former officers of Pfizer violated federal
securities laws by misrepresenting the safety of Celebrex and Bextra, and (ii) purported class actions filed by persons who claim to
be participants in the Pfizer or Pharmacia Savings Plan alleging that Pfizer and certain current and former officers, directors and
employees of Pfizer or, where applicable, Pharmacia and certain former officers, directors and employees of Pharmacia, violated
certain provisions of the Employee Retirement Income Security Act of 1974 (ERISA) by selecting and maintaining Pfizer stock or
Pharmacia stock as an investment alternative when it allegedly no longer was a suitable or prudent investment option. In June 2005,
the federal securities and ERISA actions were transferred for consolidated pre-trial proceedings to a Multi-District Litigation (In re
Pfizer Inc. Securities, Derivative and “ERISA” Litigation MDL-1688) in the U.S. District Court for the Southern District of New York.
•Securities Action in New Jersey
In 2003, several purported class action complaints were filed in the U.S. District Court for the District of New Jersey against
Pharmacia, Pfizer and certain former officers of Pharmacia. The plaintiffs seek damages, alleging that the defendants violated
federal securities laws by misrepresenting the data from a study concerning the gastrointestinal effects of Celebrex. These cases
were consolidated for pre-trial proceedings in the District of New Jersey (Alaska Electrical Pension Fund et al. v. Pharmacia
Corporation et al.). In January 2007, the court certified a class consisting of all persons who purchased Pharmacia securities from
April 17, 2000 through February 6, 2001 and were damaged as a result of the decline in the price of Pharmacia’s securities allegedly
attributable to the misrepresentations.
In October 2007, the court granted defendants’ motion for summary judgment and dismissed the plaintiffs’ claims. In November
2007, the plaintiffs appealed the decision to the U.S. Court of Appeals for the Third Circuit. In January 2009, the Third Circuit
vacated the District Court’s grant of summary judgment in favor of defendants and remanded the case to the District Court for further
proceedings. The Third Circuit also held that the District Court erred in determining that the class period ended on February 6, 2001,
and directed that the class period end on August 5, 2001. In June 2009, the District Court stayed proceedings in the case pending a
determination by the U.S. Supreme Court with regard to defendants’ petition for certiorari seeking reversal of the Third Circuit’s
decision. In May 2010, the U.S. Supreme Court denied defendants’ petition for certiorari, and the case was remanded to the District
Court for further proceedings.
•Other
Pfizer and several predecessor and affiliated companies, including Monsanto Company (Monsanto), are defendants in an action
brought by Brigham Young University (BYU) and a BYU professor in the U.S. District Court for the District of Utah alleging, among
other things, breach by Monsanto of a 1991 research agreement with BYU. Plaintiffs claim that research under that agreement led to
the discovery of Celebrex and that, as a result, they are entitled to a share of the profits from Celebrex sales. Plaintiffs seek, among
other things, compensatory and punitive damages.
Various Drugs: Off-Label Promotion Actions
•Securities Action
In May 2010, a purported class action was filed in the U.S. District Court for the Southern District of New York against Pfizer and
several of our current and former officers. The complaint alleges that the defendants violated federal securities laws by failing to
disclose that Pfizer was engaged in off-label marketing of certain drugs. Plaintiffs seek damages in an unspecified amount.
•Actions by Health Care Service Corporation
In June 2010, Health Care Service Corporation (HCSC), for itself and its affiliates, Blue Cross and Blue Shield plans in Illinois, New
Mexico, Oklahoma and Texas, filed an action against us in the U.S. District Court for the Eastern District of Texas. In July 2010,
HCSC amended its complaint. The complaint, as amended, alleges that we engaged in deceptive marketing activities, including
off-label promotion, and the payment of improper remuneration to healthcare professionals with respect to Bextra and Celebrex in
violation of, among other things, the federal Racketeer Influenced and Corrupt Organizations (RICO) Act and the Illinois Consumer
Fraud Act. In December 2010, this action was transferred to a Multi-District Litigation (In re Celebrex and Bextra Marketing, Sales
Practices and Product Liability Litigation MDL-1699) in the U.S. District Court for the Northern District of California. In July 2010,
HCSC also filed a separate lawsuit against us in the U.S. District Court for the Eastern District of Texas including substantially
2011 Financial Report 101