Pfizer 2011 Annual Report Download - page 69
Download and view the complete annual report
Please find page 69 of the 2011 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Notes to Consolidated Financial Statements
Pfizer Inc. and Subsidiary Companies
FoldRx’s lead product candidate, Vyndaqel (tafamidis meglumine), a first-in-class oral therapy for the treatment of transthyretin
familial amyloid polyneuropathy (TTR-FAP), a progressively fatal genetic neurodegenerative disease, for which liver transplant is the
only treatment option currently available, was approved in the EU in November 2011 and our new drug application was accepted for
review in the U.S. in February 2012. As a result of the November EU approval and changes in the commercial forecasts, we
increased our contingent consideration liability by approximately $85 million in 2011, with the changes recorded in Other deductions
– net.
D. Divestitures
On August 1, 2011, we completed the sale of our Capsugel business for approximately $2.4 billion in cash. In connection with the
decision to sell, the operating results associated with the Capsugel business are classified as Discontinued operations—net of tax in
the consolidated statements of income for all periods presented, and the assets and liabilities associated with this business are
classified into Assets of discontinued operations and other assets held for sale and Liabilities of discontinued operations,as
appropriate, in the consolidated balance sheets for all applicable periods presented.
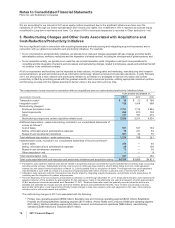
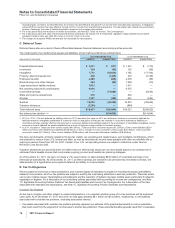
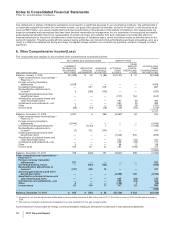
The components of Discontinued operations—net of tax, substantially all of which relate to our Capsugel business, follow:
YEAR
ENDED DECEMBER 31,
(MILLIONS OF DOLLARS) 2011 2010 2009
Revenues $ 507 $752 $740
Pre-tax (loss)/income from discontinued operations $31 $140 $148
Provision for taxes on income(a), (c) 23 52 51
Income from discontinued operations—net of tax 888 97
Pre-tax gain/(loss) on sale of discontinued operations 1,688 (11) 15
Provision/(benefit) for taxes on income(b), (d) 384 — (2)
Gain/(loss) on sale of discontinued operations—net of tax 1,304 (11) 17
Discontinued operations—net of tax $1,312 $ 77 $114
(a) Deferred tax amounts are not significant for 2011.
(b) Includes a deferred tax expense of $190 million for 2011.
(c) Includes deferred tax expense of $16 million and $8 million, respectively for 2010 and 2009.
(d) Deferred tax amounts are not significant for 2010 and 2009.
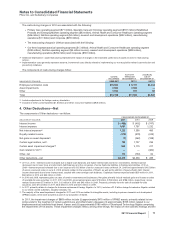
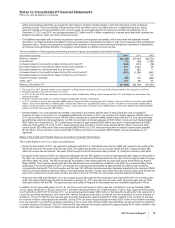
The components of Assets of discontinued operations and other assets held for sale and Liabilities of discontinued operations, most
of which relate to our Capsugel business, follow:
(MILLIONS OF DOLLARS)
YEAR ENDED
DECEMBER 31,
2010
Accounts receivable $ 186
Inventories 130
Taxes and other current assets 47
Property, plant and equipment 1,009
Goodwill 19
Identifiable intangible assets 3
Taxes and other noncurrent assets 45
Assets of discontinued operations and other assets held for sale $1,439
Current liabilities $ 124
Other liabilities 27
Liabilities of discontinued operations $ 151
The net cash flows of our discontinued operations for each of the categories of operating, investing and financing activities were not
significant for any period presented.
E. Collaborative Arrangements
In the normal course of business, we enter into collaborative arrangements with respect to in-line medicines, as well as medicines in
development that require completion of research and regulatory approval. Collaborative arrangements are contractual agreements
with third parties that involve a joint operating activity, typically a research and/or commercialization effort, where both we and our
partner are active participants in the activity and are exposed to the significant risks and rewards of the activity. Our rights and
obligations under our collaborative arrangements vary. For example, we have agreements to co-promote pharmaceutical products
discovered by us or other companies, and we have agreements where we partner to co-develop and/or participate together in
commercializing, marketing, promoting, manufacturing and/or distributing a drug product.
68 2011 Financial Report