Pfizer 2011 Annual Report Download - page 24
Download and view the complete annual report
Please find page 24 of the 2011 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
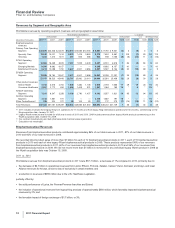
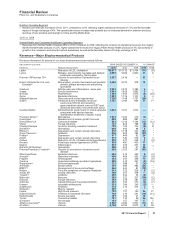
Under our co-promotion agreement with Amgen Inc. (Amgen), we co-promote Enbrel in the U.S. and Canada and share in the profits
from Enbrel sales in those countries, which we include in Alliance revenues. Our co-promotion agreement with Amgen will expire in
October 2013, and, subject to the terms of the agreement, we are entitled to a royalty stream for 36 months thereafter, which we expect
will be significantly less than our current share of Enbrel profits from U.S. and Canadian sales. Following the end of the royalty period,
we will not be entitled to any further revenues from Enbrel sales in the U.S. and Canada. Our exclusive rights to Enbrel outside the U.S.
and Canada will not be affected by the expiration of the co-promotion agreement with Amgen.
•Celebrex, indicated for the treatment of the signs and symptoms of osteoarthritis and rheumatoid arthritis worldwide and for the
management of acute pain in adults in the U.S. and certain markets in the EU, recorded increases in worldwide revenues of 6% in
2011, compared to 2010. In the U.S., revenues have been adversely affected by increased competition from generic versions of
competitive medicines and managed care formulary pressures. Celebrex is supported by continued educational and promotional efforts
highlighting its efficacy and safety profile for appropriate patients.
•Viagra remains the leading treatment for erectile dysfunction. Viagra worldwide revenues increased 3% in 2011, compared to 2010,
primarily due to the favorable impact of foreign exchange.
•Norvasc, for treating hypertension, lost exclusivity in the U.S. and other major markets in 2007 and in Canada in 2009. Norvasc
worldwide revenues decreased 4% in 2011, compared to 2010.
•Zyvox is the world’s best-selling agent among those used to treat serious Gram-positive pathogens, including methicillin-resistant
staphylococcus-aureus. Zyvox worldwide revenues increased 9% in 2011, compared to 2010, primarily due to growth in emerging
markets, as well as growth in certain other markets driven by secondary bacterial infections arising from the stronger flu season in
2011.
•Xalabrands consists of Xalatan, a prostaglandin, which is a branded agent used to reduce elevated eye pressure in patients with
open-angle glaucoma or ocular hypertension, and Xalacom, a fixed combination prostaglandin (Xalatan) and beta blocker (timolol)
available outside the U.S. Xalatan/Xalacom worldwide revenues decreased 29% in 2011, compared to 2010. Lower revenues in the
U.S. were due to the loss of exclusivity in March 2011. Lower operational revenues internationally were due to the launch of generic
latanoprost (generic Xalatan) in Japan in May 2010 and in Italy in July 2010. Xalatan and Xalacom lost exclusivity in 15 major European
markets in January 2012.
•Sutent is for the treatment of advanced renal cell carcinoma, including metastatic renal cell carcinoma (mRCC) and gastrointestinal
stromal tumors after disease progression on, or intolerance to, imatinib mesylate and advanced pancreatic neuroendocrine tumor.
Sutent worldwide revenues increased 11% in 2011, compared to 2010, due to strong operational performance and the favorable impact
of foreign exchange. We continue to drive total revenue and prescription growth, supported by cost-effectiveness data and efficacy data
in first-line mRCC––including two-year survival data, which represent the first time that overall survival of two years has been seen in
the treatment of advanced kidney cancer, as well as through increasing access and healthcare coverage. As of December 31, 2011,
Sutent was the most prescribed oral mRCC therapy in the U.S.
•Geodon/Zeldox, an atypical antipsychotic, is indicated for the treatment of schizophrenia, as monotherapy for the acute treatment of
bipolar manic or mixed episodes, and as an adjunct to lithium or valproate for the maintenance treatment of bipolar disorder. Geodon
worldwide revenues were relatively flat in 2011, compared to 2010, which reflects higher rebates in 2011 due to the impact of the U.S.
Healthcare Legislation and moderate growth in the U.S. antipsychotic market. Geodon will lose exclusivity in the U.S. in March 2012.
•Our Premarin family of products remains the leading therapy to help women address moderate-to-severe menopausal symptoms. It
recorded a decrease in worldwide revenues of 3% in 2011, compared to 2010.
•Genotropin, one of the world’s leading human growth hormones, is used in children for the treatment of short stature with growth
hormone deficiency, Prader-Willi Syndrome, Turner Syndrome, Small for Gestational Age Syndrome, Idiopathic Short Stature (in the
U.S. only) and Chronic Renal Insufficiency (outside the U.S. only), as well as in adults with growth hormone deficiency. Genotropin is
supported by a broad platform of innovative injection-delivery devices and patient-support programs. Genotropin worldwide revenues
were relatively flat in 2011, compared to 2010.
•Detrol/Detrol LA, a muscarinic receptor antagonist, is one of the most prescribed branded medicines worldwide for overactive bladder.
Detrol LA is an extended-release formulation taken once a day. Detrol/Detrol LA worldwide revenues declined 13% in 2011, compared
to 2010, primarily due to increased competition from other branded medicines and a shift in promotional focus to our Toviaz product in
most major markets. Detrol immediate release (Detrol IR) will lose exclusivity in the U.S. in September 2012.
•Vfend is a broad-spectrum agent for treating yeast and molds. Vfend worldwide revenues decreased 9% in 2011, compared to 2010.
While international revenues of Vfend continued to be driven in 2011 by its acceptance as an excellent broad-spectrum agent for
treating serious yeast and molds, revenues in the U.S. declined primarily due to a loss of exclusivity of Vfend tablets and the launch of
generic voriconazole (generic Vfend) in February 2011.
•Chantix/Champix is an aid to smoking-cessation treatment in adults 18 years of age and older. Chantix/Champix worldwide revenues
decreased 5% in 2011, compared to 2010. Revenues in 2011 were favorably impacted by foreign exchange, which was more than
offset by the impact of changes to the product’s label and other factors. We are continuing our educational and promotional efforts,
which are focused on addressing the significant health consequences of smoking highlighting the Chantix benefit-risk proposition and
emphasizing the importance of the physician-patient dialogue in helping patients quit smoking.
In July 2011, the U.S. prescribing information was revised to include clinical data showing that Chantix is an effective aid to
smoking-cessation treatment for smokers with stable cardiovascular disease (CVD) and mild-to-moderate chronic obstructive
pulmonary disease (COPD). The revised label also includes a warning/precaution advising smokers with CVD to inform their
physician of any new or worsening symptoms of cardiovascular disease, and to seek emergency medical help if they experience
any symptoms of a heart attack.
2011 Financial Report 23