Pfizer 2011 Annual Report Download - page 20
Download and view the complete annual report
Please find page 20 of the 2011 Pfizer annual report below. You can navigate through the pages in the report by either clicking on the pages listed below, or by using the keyword search tool below to find specific information within the annual report.
Financial Review
Pfizer Inc. and Subsidiary Companies
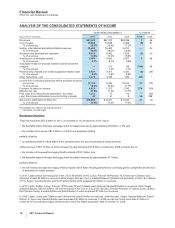
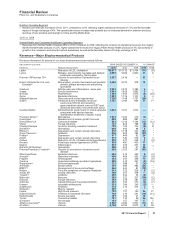
Geographically,
•in the U.S., revenues from biopharmaceutical products decreased 9% in 2011, compared to 2010, reflecting lower revenues from
Lipitor, Protonix, Effexor, Zosyn, Xalatan, Vfend, Caduet and Aromasin, all due to loss of exclusivity, lower Alliance revenues due to
loss of exclusivity of Aricept 5mg and 10mg tablets in November 2010 and lower revenues from Detrol/Detrol LA, as well as the
reduction in revenues of $359 million in 2011 due to the U.S. Healthcare Legislation. The impact of these adverse factors was partially
offset by the strong performance of certain other biopharmaceutical products and the addition of U.S. revenues from legacy King
products of approximately $904 million in 2011.
•in our international markets, revenues from biopharmaceutical products increased 5% in 2011, compared to 2010, reflecting the
favorable impact of foreign exchange of 6% in 2011, partially offset by a net operational decrease. Operationally, revenues were
favorably impacted by increases in the Prevenar franchise, Lyrica, Enbrel, Celebrex and Alliance revenues and unfavorably impacted
by declines in Lipitor, Effexor, Norvasc and Xalatan/Xalacom. International revenues from legacy King products were not significant to
our international revenues in 2011.
During 2011, international revenues from biopharmaceutical products represented 59% of total revenues from biopharmaceutical
products, compared to 56% in 2010.
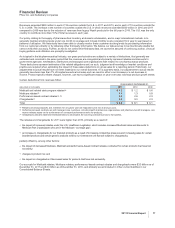
Primary Care Operating Segment
•Primary Care unit revenues decreased 3% in 2011 compared to 2010, due to lower operational revenues of 6%, partially offset by the
favorable impact of foreign exchange of 3%. Primary Care unit revenues were favorably impacted by higher revenues from certain
patent-protected products, including Lyrica, Celebrex, Pristiq and Spiriva (in Alliance revenues), among others, as well as the addition
of revenues from legacy King products of $404 million, or 2% in 2011. Operational revenues in 2011 were negatively impacted by the
loss of exclusivity of Lipitor and Caduet in the U.S. in November 2011, Lipitor in various other developed markets during 2010, as well
as Aricept 5mg and 10 mg tablets in the U.S. in November 2010. Taken together, these losses of exclusivity reduced Primary Care unit
revenues by approximately $2.1 billion, or 9%, in comparison 2010.
Specialty Care and Oncology Operating Segment
•Specialty Care unit revenues increased 1% compared to 2010 due to the favorable impact of foreign exchange of 3%, partially offset by
lower operational revenues of 2%. Operational revenues were favorably impacted by strong growth in the Prevnar/Prevenar franchise
and Enbrel, and unfavorably impacted by the loss of exclusivity of Vfend and Xalatan in the U.S. in February and March 2011,
respectively. Collectively, these losses of exclusivity reduced Specialty Care unit revenues by $624 million, or 4%, in comparison with
2010.
•Oncology unit revenues decreased 6%, compared to 2010, due to lower operational revenues of 10%, partially offset by the favorable
impact of foreign exchange of 4%. The decrease in the Oncology unit operational revenues in 2011 was primarily due to the transfer of
Aromasin’s U.S. business to the Established Products unit effective January 1, 2011 as a result of its loss of exclusivity in April 2011.
This loss of exclusivity reduced Oncology unit revenues by $160 million, or 11%, in comparison with 2010.
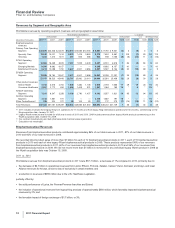
Established Products and Emerging Markets Operating Segment
•Established Products unit revenues decreased 9% in 2011 compared to 2010 due to lower operational revenues of 13%, partially offset
by a 4% favorable impact of foreign exchange. The decrease in Established Products unit operational revenues in 2011 was mainly
due to the loss of exclusivity of Effexor XR, Protonix and Zosyn in the U.S. Taken together, these losses of exclusivity decreased
Established Products unit revenues by $1.7 billion, or 17%, in comparison with 2010. These declines were partially offset by the
addition of revenues from legacy King products of $546 million, or 5% in 2011.
•Emerging Markets unit revenues increased 7%, compared to 2010, due to higher operational revenues of 5%, as well as a 2%
favorable impact of foreign exchange. The increase in Emerging Markets unit operational revenues in 2011 was due to growth in
certain key innovative brands, primarily the Prevenar franchise, Lyrica, Enbrel, Celebrex, Vfend and Zyvox. These increases were
partially offset by lower revenues from Lipitor, which lost exclusivity in Brazil in August 2010 and Mexico in December 2010, as well as
the impact of price reductions for certain products in certain emerging market countries. These losses of exclusivity reduced Emerging
Market unit revenues by $118 million, or 1%, in comparison with 2010.
Total revenues from established products in both the Established Products and Emerging Markets units were $13.0 billion, with $3.8
billion generated in emerging markets in 2011.
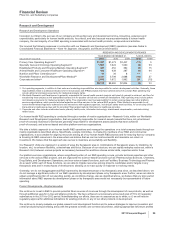
2010 vs. 2009
Worldwide revenues from biopharmaceutical products in 2010 were $58.5 billion, an increase of 29% compared to 2009, primarily
due to:
•the inclusion of operational revenues from legacy Wyeth products of approximately $13.7 billion, which favorably impacted
biopharmaceutical revenues by 30%; and
•the weakening of the U.S. dollar relative to other currencies, primarily the Canadian dollar, Australian dollar, Japanese yen and
Brazilian real, which favorably impacted biopharmaceutical revenues by approximately $900 million, or 2%,
partially offset by:
•the decrease in operational revenues of approximately $1.5 billion, or 3%, from legacy Pfizer products overall, including Norvasc,
Camptosar, Lipitor and Detrol/Detrol LA.
2011 Financial Report 19